Extracellular vesicles of Bacteroides uniformis induce M1 macrophage polarization and aggravate gut inflammation during weaning
IF 7.9
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Weaning process is commonly associated with gastrointestinal inflammation and dysbiosis of the intestinal microbes. In particular, the impact of gut bacteria and extracellular vesicles on the etiology of intestinal inflammation during weaning is not well understood. We have uncovered a potential link between gut inflammation and the corresponding variation of macrophage bacterial sensing and pro-inflammatory polarization during the weaning process of piglets through single-cell transcriptomic analyses. We conducted a comprehensive analysis of bacterial distribution across the gastrointestinal tract and pinpointed Bacteroides uniformis enriching in piglets undergoing weaning. Next, we found out that exposure to B. uniformis-derived extracellular vesicles (BEVs) exacerbated gut inflammation in a murine colitis model while recruiting and polarizing intestinal macrophages toward a pro-inflammatory phenotype. BEVs modulated the function of macrophages cultured in vitro by suppressing the granulocyte-macrophage colony-stimulating factor/signal transducer and activator of transcription 5/arginase 1 pathway, thereby affecting polarization toward an M1-like state. The effects of BEVs were verified both in the macrophage clearance murine model and by using an adoptive transfer assay. Our findings highlight the involvement of BEVs in facilitating the polarization of pro-inflammatory macrophages and promoting gut inflammation during weaning.
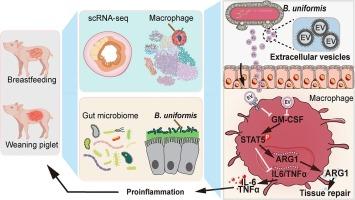
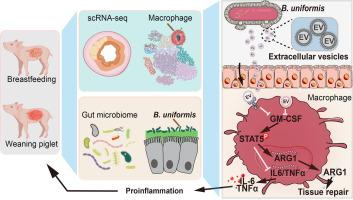
均匀乳杆菌的胞外囊泡会诱导 M1 巨噬细胞极化,并加剧断奶期间的肠道炎症。
断奶过程通常与胃肠道炎症和肠道微生物菌群失调有关。尤其是肠道细菌和细胞外囊泡(EV)对断奶期间肠道炎症病因的影响尚不十分清楚。我们通过单细胞转录组分析发现了仔猪断奶过程中肠道炎症与巨噬细胞细菌感应和促炎极化的相应变化之间的潜在联系。我们对整个胃肠道的细菌分布进行了全面分析,发现均匀乳杆菌(B. uniformis)在断奶仔猪中富集。接下来,我们发现,在小鼠结肠炎模型中,暴露于均匀杆菌衍生的EVs(BEVs)会加剧肠道炎症,同时招募肠道巨噬细胞并使其极化为促炎表型。BEVs 通过抑制 GM-CSF/STAT5/ARG1 通路调节体外培养巨噬细胞的功能,从而影响向 M1 样态的极化。BEVs的作用在巨噬细胞清除小鼠模型中和通过采用转移试验都得到了验证。我们的研究结果突出表明,BEVs 参与促进促炎巨噬细胞的极化,并在断奶期间促进肠道炎症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


