Past, present, and future of cell replacement therapy for parkinson’s disease: a novel emphasis on host immune responses
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
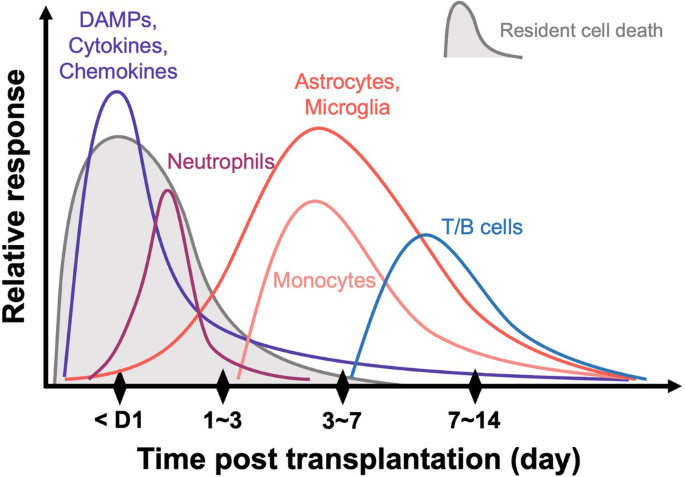
Parkinson’s disease (PD) stands as the second most common neurodegenerative disorder after Alzheimer’s disease, and its prevalence continues to rise with the aging global population. Central to the pathophysiology of PD is the specific degeneration of midbrain dopamine neurons (mDANs) in the substantia nigra. Consequently, cell replacement therapy (CRT) has emerged as a promising treatment approach, initially supported by various open-label clinical studies employing fetal ventral mesencephalic (fVM) cells. Despite the initial favorable results, fVM cell therapy has intrinsic and logistical limitations that hinder its transition to a standard treatment for PD. Recent efforts in the field of cell therapy have shifted its focus towards the utilization of human pluripotent stem cells, including human embryonic stem cells and induced pluripotent stem cells, to surmount existing challenges. However, regardless of the transplantable cell sources (e.g., xenogeneic, allogeneic, or autologous), the poor and variable survival of implanted dopamine cells remains a major obstacle. Emerging evidence highlights the pivotal role of host immune responses following transplantation in influencing the survival of implanted mDANs, underscoring an important area for further research. In this comprehensive review, building upon insights derived from previous fVM transplantation studies, we delve into the functional ramifications of host immune responses on the survival and efficacy of grafted dopamine cells. Furthermore, we explore potential strategic approaches to modulate the host immune response, ultimately aiming for optimal outcomes in future clinical applications of CRT for PD.
帕金森病细胞替代疗法的过去、现在和未来:对宿主免疫反应的新强调。
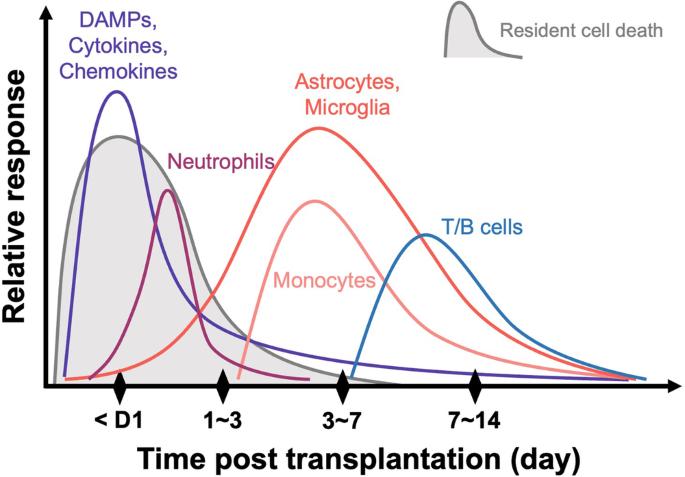
帕金森病(PD)是仅次于阿尔茨海默病的第二大常见神经退行性疾病,其发病率随着全球人口老龄化而持续上升。帕金森病病理生理学的核心是黑质中脑多巴胺神经元(mDANs)的特异性变性。因此,细胞替代疗法(CRT)已成为一种很有前景的治疗方法,最初得到了采用胎儿腹腔间脑(fVM)细胞的各种开放标签临床研究的支持。尽管胎儿腹腔间脑细胞疗法取得了初步的良好效果,但其内在和后勤方面的局限性阻碍了该疗法向治疗帕金森病的标准疗法过渡。最近,细胞疗法领域的工作重点已转向利用人类多能干细胞,包括人类胚胎干细胞和诱导多能干细胞,以克服现有的挑战。然而,无论移植细胞来源如何(如异种、异体或自体),植入的多巴胺细胞存活率低且不稳定仍是一大障碍。新出现的证据突显了移植后宿主免疫反应在影响植入的多巴胺细胞存活方面的关键作用,强调了进一步研究的重要领域。在这篇综合综述中,我们以先前的 fVM 移植研究为基础,深入探讨了宿主免疫反应对移植多巴胺细胞存活和疗效的功能性影响。此外,我们还探讨了调节宿主免疫反应的潜在策略方法,最终目标是在未来治疗帕金森病的 CRT 临床应用中取得最佳效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


