Edge sites dominate the hydrogen evolution reaction on platinum nanocatalysts
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Platinum nanocatalysts facilitate the hydrogen evolution reaction (HER) for renewable chemical fuel generation. These nanostructures encompass diverse surface sites, including (111) and (100) facets and edge sites between them. Identifying the exact active sites is essential for optimal catalyst design, but remains challenging. Here, combining electrical transport spectroscopy (ETS) with reactive force field (ReaxFF) calculations, we profile hydrogen adsorption on platinum nanowires and reveal two distinct peaks: one at 0.20 VRHE for (111) and (100) facets and one at 0.038 VRHE for edge sites. Concurrent ETS and cyclic voltammetry show that edge site adsorption coincides with the onset of the HER, indicating the critical role of edge sites. ReaxFF molecular dynamics calculations confirm lower activation barriers for the HER at edge sites, with two to four orders of magnitude higher turnover frequencies. ETS in alkaline media reveals substantially suppressed hydrogen adsorption on edge sites, contributing to the more sluggish HER kinetics. These findings resolve the elusive role of different sites on platinum surfaces, offering critical insights for HER catalyst design. Pt is the most active catalyst for the hydrogen evolution reaction in acidic media, but the precise nature of its active sites remains elusive. Now electrical transport spectroscopy and molecular dynamics are combined to map the hydrogen adsorption sites on Pt nanowires and reveal the much higher activity of (111)/(100) edge sites.
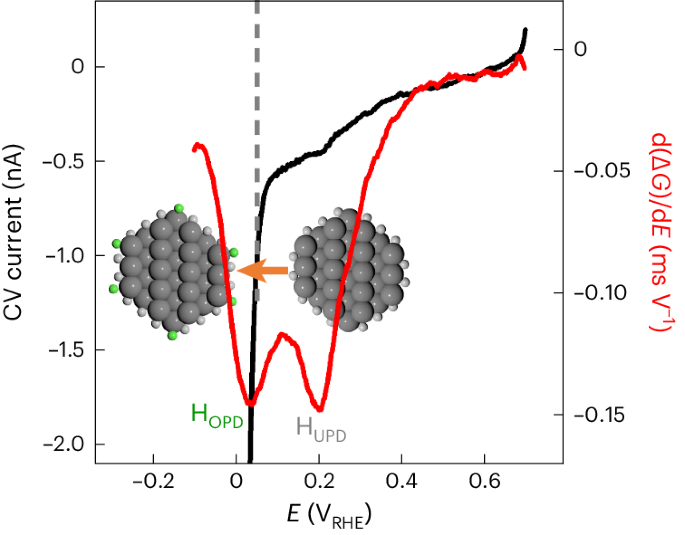
铂纳米催化剂上的氢进化反应以边缘位为主
铂纳米催化剂可促进氢进化反应(HER),从而产生可再生化学燃料。这些纳米结构包含不同的表面位点,包括(111)和(100)面以及它们之间的边缘位点。确定确切的活性位点对于优化催化剂设计至关重要,但仍具有挑战性。在这里,我们将电迁移光谱(ETS)与反应力场(ReaxFF)计算相结合,对铂纳米线上的氢吸附进行了剖析,并发现了两个不同的峰值:一个在 0.20 VRHE 处为(111)和(100)面,另一个在 0.038 VRHE 处为边缘位点。同时进行的 ETS 和循环伏安分析表明,边缘位点的吸附与 HER 的发生相吻合,这表明边缘位点起着关键作用。ReaxFF 分子动力学计算证实,边缘位点的 HER 激活障碍较低,翻转频率高出 2 到 4 个数量级。碱性介质中的 ETS 显示,边缘位点上的氢吸附受到了极大的抑制,从而导致 HER 动力学更加缓慢。这些发现解决了铂表面不同位点难以捉摸的作用问题,为 HER 催化剂的设计提供了重要的启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


