Efficacy and safety of first-line regimens for advanced HER2-positive breast cancer: A Bayesian network meta-analysis
Abstract
Background
The current standard of care for advanced human epidermal growth factor receptor 2 (HER2)-positive breast cancer is pertuzumab plus trastuzumab and docetaxel as first-line therapy. However, with the development of newer treatment regimens, there is a lack of evidence regarding which is the optimal treatment strategy. The aim of this network meta-analysis was to evaluate the efficacy and safety of first-line regimens for advanced HER2-positive breast cancer by indirect comparisons.
Methods
A systematic review and Bayesian network meta-analysis were conducted. The PubMed, EMBASE, and Cochrane Library databases were searched for relevant articles published through to December 2023. The hazard ratio (HR) and 95% credible interval (CrI) were used to compare progression-free survival (PFS) between treatments, and the odds ratio and 95% CrI were used to compare the objective response rate (ORR) and safety.
Results
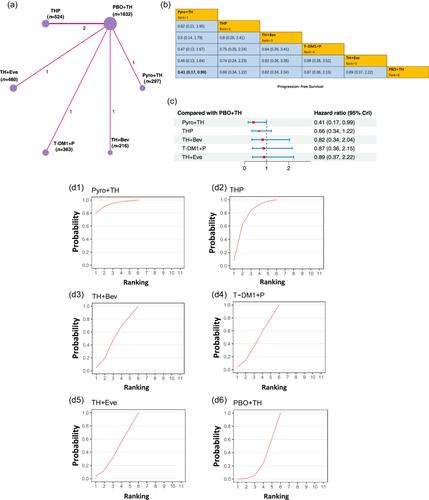
Twenty randomized clinical trials that included 15 regimens and 7094 patients were analyzed. Compared with the traditional trastuzumab and docetaxel regimen, PFS was longer on the pyrotinib and trastuzumab plus docetaxel regimen (HR: 0.41, 95% CrI: 0.22–0.75) and the pertuzumab and trastuzumab plus docetaxel regimen (HR: 0.65, 95% CrI: 0.43–0.98). Consistent with the results for PFS, the ORR was better on the pyrotinib and trastuzumab plus docetaxel regimen and the pertuzumab and trastuzumab plus docetaxel regimen than on the traditional trastuzumab and docetaxel regimen. The surface under the cumulative ranking curve indicated that the pyrotinib and trastuzumab plus docetaxel regimen was most likely to rank first in achieving the best PFS and ORR. Comparable results were found for grade ≥3 AE rates of ≥10%.
Conclusions
Our results suggest that the pyrotinib and trastuzumab plus docetaxel regimen is most likely to be the optimal first-line therapy for patients with HER2-positive breast cancer.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


