Five undescribed drimane-type sesquiterpenes from Polygonum hydropiper
IF 1.3
4区 生物学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
In order to find various structural and active compounds, 95 % ethanol extract of P. hydropiper was investigated to obtain five undescribed drimane sesquiterpenes 3-O-angeloyl-11-O-methyldanilol (1), 3-O-tigloyl-11-O-methyldanilol (2), 3-O-(3′-methylbutenoyl)-11-O-methyldanilol (3), 3-O-(2′-metylbutanoyl)-11-O-methyldanilol (4) and 2α, 3β-ditigloyloxy-11-O-methylisodrimeninol (5). Their structures were established by extensive spectroscopic analyses. Furthermore, 3-O-angeloyl-11-O-methyldanilol (1) showed moderate anti-platelet aggregation activity according to in vitro pharmacological evaluation.
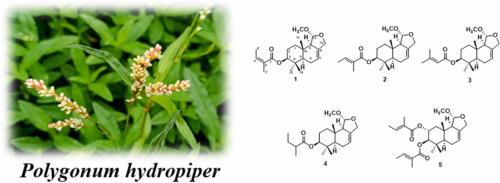
何首乌中五种未被描述的 drimane 型倍半萜类化合物
hydropiper 的 95 % 乙醇提取物,获得了五种未曾描述过的 drimane 倍半萜 3-O-angeloyl-11-O-methyldanilol (1)、3-O-tigloyl-11-O-methyldanilol (2)、3-O-(3′-methylbutenoyl)-11-O-methyldanilol (3)、3-O-(2′-metylbutanoyl)-11-O-methyldanilol (4) 和 2α,3β-ditigloyloxy-11-O-methylisodrimeninol (5)。通过大量的光谱分析确定了它们的结构。此外,根据体外药理评估,3-O-天使酰基-11-O-甲基二苯胺醇(1)显示出中等程度的抗血小板聚集活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry Letters
生物-生化与分子生物学
CiteScore
3.00
自引率
11.80%
发文量
190
审稿时长
34 days
期刊介绍:
Phytochemistry Letters invites rapid communications on all aspects of natural product research including:
• Structural elucidation of natural products
• Analytical evaluation of herbal medicines
• Clinical efficacy, safety and pharmacovigilance of herbal medicines
• Natural product biosynthesis
• Natural product synthesis and chemical modification
• Natural product metabolism
• Chemical ecology
• Biotechnology
• Bioassay-guided isolation
• Pharmacognosy
• Pharmacology of natural products
• Metabolomics
• Ethnobotany and traditional usage
• Genetics of natural products
Manuscripts that detail the isolation of just one new compound are not substantial enough to be sent out of review and are out of scope. Furthermore, where pharmacology has been performed on one new compound to increase the amount of novel data, the pharmacology must be substantial and/or related to the medicinal use of the producing organism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


