Coupling enzymatic activity and gating in an ancient TRPM chanzyme and its molecular evolution
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
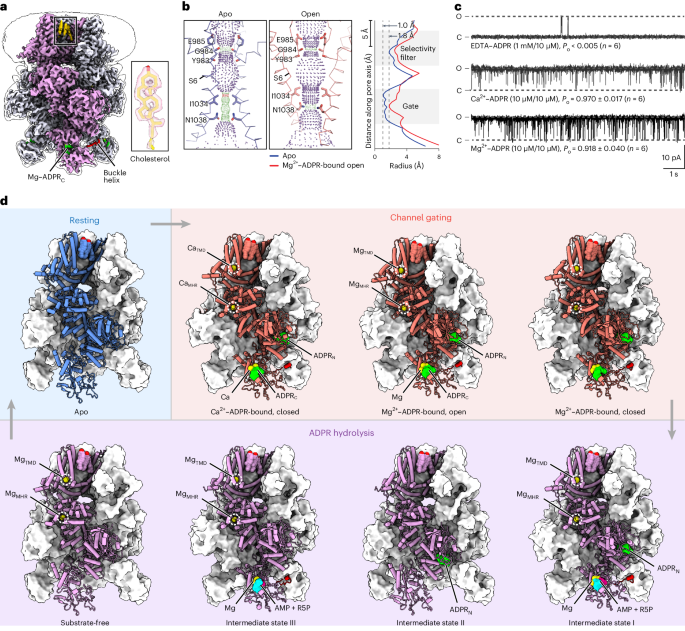
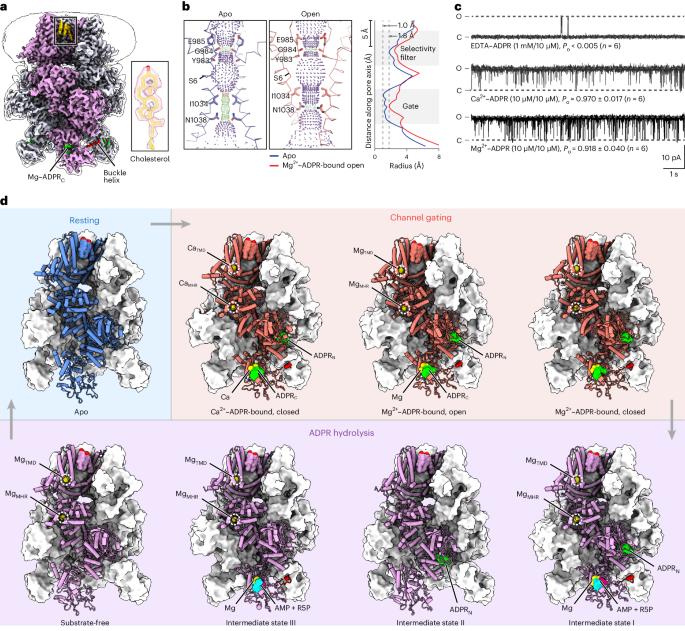
Channel enzymes represent a class of ion channels with enzymatic activity directly or indirectly linked to their channel function. We investigated a TRPM2 chanzyme from choanoflagellates that integrates two seemingly incompatible functions into a single peptide: a channel module activated by ADP-ribose with high open probability and an enzyme module (NUDT9-H domain) consuming ADP-ribose at a remarkably slow rate. Using time-resolved cryogenic-electron microscopy, we captured a complete series of structural snapshots of gating and catalytic cycles, revealing the coupling mechanism between channel gating and enzymatic activity. The slow kinetics of the NUDT9-H enzyme module confers a self-regulatory mechanism: ADPR binding triggers NUDT9-H tetramerization, promoting channel opening, while subsequent hydrolysis reduces local ADPR, inducing channel closure. We further demonstrated how the NUDT9-H domain has evolved from a structurally semi-independent ADP-ribose hydrolase module in early species to a fully integrated component of a gating ring essential for channel activation in advanced species. Using time-resolved cryo-EM, the authors capture complete structural snapshots of the enzymatic cycle coupled with channel gating in a TRPM-type channel enzyme.
古老的 TRPM 酶的酶活性和门控耦合及其分子进化
通道酶代表了一类具有与其通道功能直接或间接相关的酶活性的离子通道。我们研究了一种来自鹅鞭毛虫的 TRPM2 通道酶,它将两种看似不相容的功能整合到了一条肽中:一个通道模块由 ADP-ribose 激活,具有高开放概率;一个酶模块(NUDT9-H 结构域)以极慢的速度消耗 ADP-ribose。利用时间分辨低温电子显微镜,我们捕捉到了一系列完整的门控和催化循环结构快照,揭示了通道门控和酶活性之间的耦合机制。NUDT9-H 酶模块的缓慢动力学赋予了一种自我调节机制:ADPR 结合触发 NUDT9-H 四聚体,促进通道开放,而随后的水解减少了局部 ADPR,诱导通道关闭。我们进一步证明了 NUDT9-H 结构域是如何从早期物种中结构上半独立的 ADP 核糖水解酶模块进化为高级物种中通道激活所必需的门控环的完全整合元件的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


