Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
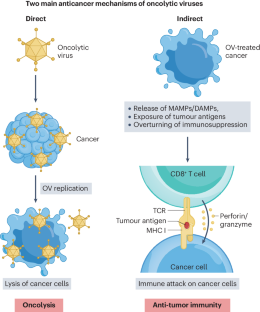
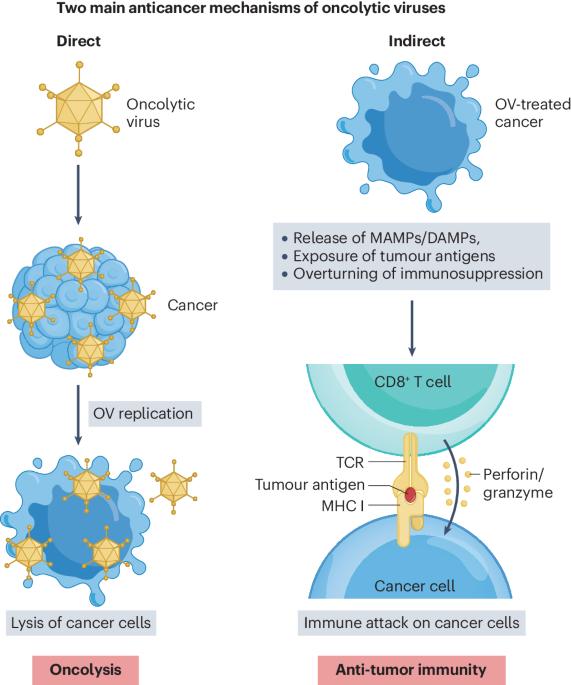
Oncolytic viruses (OVs) represent a novel class of cancer immunotherapy agents that preferentially infect and kill cancer cells and promote protective antitumor immunity. Furthermore, OVs can be used in combination with established or upcoming immunotherapeutic agents, especially immune checkpoint inhibitors, to efficiently target a wide range of malignancies. The development of OV-based therapy involves three major steps before clinical evaluation: design, production and preclinical testing. OVs can be designed as natural or engineered strains and subsequently selected for their ability to kill a broad spectrum of cancer cells rather than normal, healthy cells. OV selection is further influenced by multiple factors, such as the availability of a specific viral platform, cancer cell permissivity, the need for genetic engineering to render the virus non-pathogenic and/or more effective and logistical considerations around the use of OVs within the laboratory or clinical setting. Selected OVs are then produced and tested for their anticancer potential by using syngeneic, xenograft or humanized preclinical models wherein immunocompromised and immunocompetent setups are used to elucidate their direct oncolytic ability as well as indirect immunotherapeutic potential in vivo. Finally, OVs demonstrating the desired anticancer potential progress toward translation in patients with cancer. This tutorial provides guidelines for the design, production and preclinical testing of OVs, emphasizing considerations specific to OV technology that determine their clinical utility as cancer immunotherapy agents. This tutorial provides guidelines on oncolytic virus design, production and testing in cancer immunotherapy. Best practice recommendations for preclinical and clinical use of oncolytic viruses as an immunotherapy tool and related future challenges are also considered.
教程:用于癌症免疫疗法的溶瘤病毒的设计、生产和测试。
肿瘤溶解病毒(OV)是一类新型的癌症免疫治疗药物,它能优先感染和杀死癌细胞,并促进保护性抗肿瘤免疫。此外,OVs 还可与现有或即将推出的免疫治疗药物(尤其是免疫检查点抑制剂)联合使用,有效地针对各种恶性肿瘤。开发基于 OV 的疗法涉及临床评估前的三个主要步骤:设计、生产和临床前测试。OV 可以设计为天然菌株或工程菌株,然后根据其杀死广谱癌细胞而非正常健康细胞的能力进行筛选。OV 的选择还受到多种因素的影响,如特定病毒平台的可用性、癌细胞允许性、是否需要通过基因工程使病毒不致病和/或更有效,以及在实验室或临床环境中使用 OV 的后勤考虑因素。然后,利用免疫功能低下和免疫功能正常的临床前模型,生产和测试选定的 OVs 的抗癌潜力,以阐明它们在体内的直接溶瘤能力和间接免疫治疗潜力。最后,具有理想抗癌潜力的 OV 将在癌症患者身上得到应用。本教程为 OV 的设计、生产和临床前测试提供指导,强调了 OV 技术的具体注意事项,这些注意事项决定了 OV 作为癌症免疫疗法药物的临床用途。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


