Discovery of N–X anomeric amides as electrophilic halogenation reagents
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
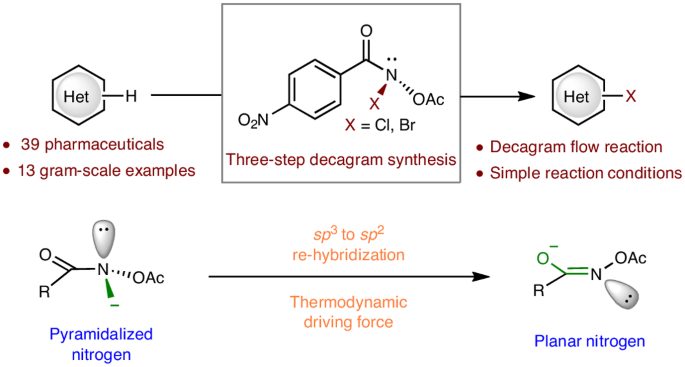
Electrophilic halogenation is a widely used tool employed by medicinal chemists to either pre-functionalize molecules for further diversity or incorporate a halogen atom into drugs or drug-like compounds to solve metabolic problems or modulate off-target effects. Current methods to increase the power of halogenation rely on either the invention of new reagents or activating commercially available reagents with various additives such as Lewis or Brønsted acids, Lewis bases and hydrogen-bonding activators. There is a high demand for new reagents that can halogenate otherwise unreactive compounds under mild conditions. Here we report the invention of a class of halogenating reagents based on anomeric amides, taking advantage of the energy stored in the pyramidalized nitrogen of N–X anomeric amides as a driving force. These robust halogenating methods are compatible with a variety of functional groups and heterocycles, as exemplified on over 50 compounds (including 13 gram-scale examples and 1 flow chemistry scale-up). Electrophilic halogenation approaches often suffer from low reactivity and chemoselectivity when it comes to complex compounds. Now a class of halogenating reagents based on anomeric amides that can halogenate complex bioactive molecules with diverse functional groups and heterocycles has been developed. The higher reactivity of these anomeric amide reagents is attributed to the energy stored in the pyramidalized nitrogen.
发现作为亲电卤化试剂的 N-X 异构体酰胺
亲电卤化是药物化学家广泛使用的一种工具,可以预官能化分子,使其进一步多样化,或将卤原子加入药物或类药物中,以解决代谢问题或调节脱靶效应。目前提高卤化能力的方法要么依赖于发明新试剂,要么依赖于用各种添加剂(如路易斯酸或布伦斯特酸、路易斯碱和氢键活化剂)激活市售试剂。目前,人们对能够在温和条件下卤化原本不反应的化合物的新试剂需求量很大。在此,我们报告了一类基于同分异构酰胺的卤化试剂的发明,该试剂利用了 N-X 同分异构酰胺的金字塔化氮中储存的能量作为驱动力。这些稳健的卤化方法与各种官能团和杂环兼容,在 50 多种化合物(包括 13 个克级实例和 1 个流式化学放大实例)上得到了验证。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


