Integrative analyses identified gap junction beta-2 as a prognostic biomarker and therapeutic target for breast cancer
Abstract
Background
Increasing evidence has shown that connexins are involved in the regulation of tumor development, immune escape, and drug resistance. This study investigated the gene expression patterns, prognostic values, and potential mechanisms of connexins in breast cancer.
Methods
We conducted a comprehensive analysis of connexins using public gene and protein expression databases and clinical samples from our institution. Connexin mRNA expressions in breast cancer and matched normal tissues were compared, and multiomics studies were performed.
Results
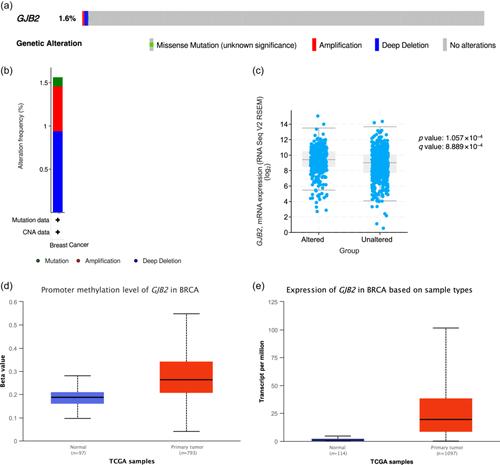
Gap junction beta-2 mRNA was overexpressed in breast cancers of different pathological types and molecular subtypes, and its high expression was associated with poor prognosis. The tumor membrane of the gap junction beta-2 mutated group was positive, and the corresponding protein was expressed. Somatic mutation and copy number variation of gap junction beta-2 are rare in breast cancer. The gap junction beta-2 transcription level in the p110α subunit of the phosphoinositide 3-kinase mutant subgroup was higher than that in the wild-type subgroup. Gap junction beta-2 was associated with the phosphoinositide 3-kinase-Akt signaling pathway, extracellular matrix–receptor interaction, focal adhesion, and proteoglycans in cancer. Furthermore, gap junction beta-2 overexpression may be associated with phosphoinositide 3-kinase and histone deacetylase inhibitor resistance, and its expression level correlated with infiltrating CD8+ T cells, macrophages, neutrophils, and dendritic cells.
Conclusions
Gap junction beta-2 may be a promising therapeutic target for targeted therapy and immunotherapy and may be used to predict breast cancer prognosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


