Procedural and clinical outcomes of patients undergoing a TAVI in TAVI procedure: Rationale and design of the multicentre, prospective, observational ReTAVI registry
Abstract
Background
Transcatheter aortic valve implantation (TAVI) is increasingly being used in younger patients and those with lower peri-procedural risk, meaning more patients will live long enough to experience structural valve deterioration (SVD) of the bioprosthesis, indicating repeated TAVI. Experience of repeated TAVI—transcatheter heart valve (THV) implantation into an index THV is limited. This registry aims to assess the peri-procedural and short-term safety, efficacy and durability of repeated TAVI.
Methods
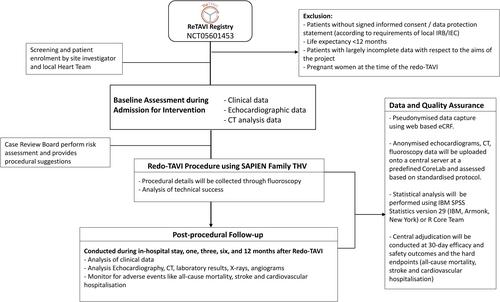
The ReTAVI Prospective observational registry is an investigator-initiated, multicentre, international, prospective registry of patients undergoing repeated TAVI using balloon-expandable SAPIEN prosthesis to evaluate procedural and short-term safety, efficacy and durability as well as anatomical and procedural factors associated with optimal results. The registry will enrol at least 150 patients across 60 high-volume centres. Patients must be ≥18 years old, have had procedural success with their first TAVI, have index THV device failure, intend to undergo repeated TAVI and be considered suitable candidates by their local Heart Team. All patients will undergo a 30-day and 12-month follow-up. The estimated study completion is 2025.
Conclusions
The registry will collect pre-, peri-, postoperative and 12-months data on patients undergoing repeated TAVI procedures with THVs for failure of the index THV and determine VARC-3-defined efficacy and safety at 30 days and functional outcome at 12 months. The registry will expand existing data sets and identify patient characteristics/indicators related to complications and clinical benefits for patients with symptomatic severe calcific degenerative aortic stenosis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


