Transcriptomics reveals transient and dynamic muscle fibrosis and atrophy differences following spinal cord injury in rats
Abstract
Background
The rate and magnitude of skeletal muscle wasting after severe spinal cord injury (SCI) exceeds most other disuse conditions. Assessing the time course of molecular changes can provide insight into the progression of muscle wasting post-SCI. The goals of this study were (1) to identify potential targets that may prevent the pathologic features of SCI in soleus muscles and (2) to establish therapeutic windows for treating these pathologic changes.
Methods
Four-month-old Sprague–Dawley male rats received T9 laminectomy (SHAM surgery) or severe contusion SCI. Hindlimb locomotor function was assessed weekly, with soleus muscles obtained 1 week, 2 weeks, 1 month and 3 months post-surgery (n = 6–7 per group per timepoint). RNA was extracted from muscles for bulk RNA-sequencing analysis (n = 3–5 per group per timepoint). Differentially expressed genes (DEGs) were evaluated between age-matched SHAM and SCI animals. Myofiber size, muscle fibre type and fibrosis were assessed on contralateral muscles.
Results
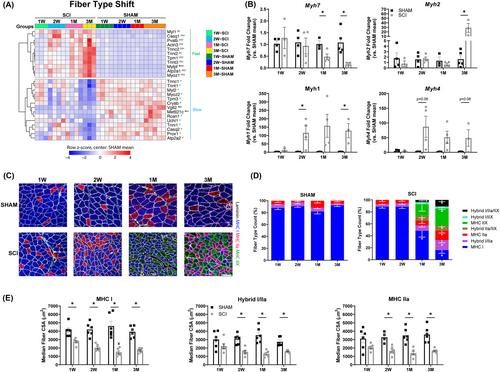
SCI produced immediate and persistent hindlimb paralysis, with Basso–Beattie–Bresnahan locomotor scores remaining below 7 throughout the study, contributing to a progressive 25–50% lower soleus mass and myofiber atrophy versus SHAM (P < 0.05 at all timepoints). Transcriptional comparisons of SCI versus SHAM resulted in 184 DEGs (1 week), 436 DEGs (2 weeks), 133 DEGs (1 month) and 1200 DEGs (3 months). Upregulated atrophy-related genes included those associated with cell senescence, nuclear factor kappa B, ubiquitin proteasome and unfolded protein response pathways, along with upregulated genes that negatively influence muscle growth through the transforming growth factor beta pathway and inhibition of insulin-like growth factor-I/Akt/mechanistic target of rapamycin and p38/mitogen-activated protein kinase signalling. Genes associated with extracellular matrix (ECM), including collagens, collagen crosslinkers, proteoglycans and those regulating ECM integrity, were enriched within upregulated DEGs at 1 week but subsequently downregulated at 2 weeks and 3 months and were accompanied by >50% higher ECM areas and hydroxyproline levels in SCI muscles (P < 0.05). Myofiber remodelling genes were enriched in upregulated DEGs at 2 weeks and 1 month and were downregulated at 3 months. Genes that regulate neuromuscular junction remodelling were evident in muscles post-SCI, along with slow-to-fast fibre-type shifts: 1 week and 2 weeks SCI muscles were composed of 90% myosin heavy chain (MHC) type I fibres, which decreased to only 16% at 3 months and were accompanied by 50% fibres containing MHC IIX (P < 0.05). Metabolism genes were enriched in upregulated DEGs at 1 month and were further enriched at 3 months.
Conclusions
Our results substantiate many known pathologic features of SCI-induced wasting in rat skeletal muscle and identify a progressive and dynamic transcriptional landscape within the post-SCI soleus. Future studies are warranted to consider these therapeutic treatment windows when countering SCI muscle pathology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


