Mildly preheating induced conformational changes of soy protein isolates contributed to the binding interaction with blueberry anthocyanins for stabilization
Abstract
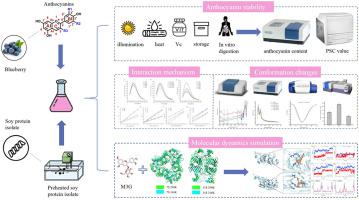
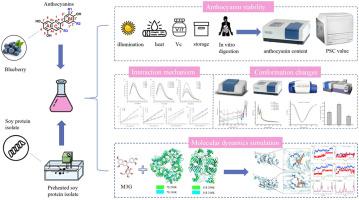
Preheated proteins have been proven to stabilize anthocyanins better than natural proteins, but the binding mechanism remains to be further studied. This study investigated the interacting mechanism between soy protein isolate (SPI) that has been slightly heated and blueberry anthocyanins (ANs) using multi-spectroscopy and molecular dynamics approaches. It was found that preheated SPI (pre-SPI) increased the stability of the ANs and provided better protection than natural SPI. In particular, SPI preheated at 45 °C provided the most effective protection against anthocyanin instability. Multi-spectroscopy demonstrates that SPI exhibits a robust affinity for and causes static quenching of malvidin-3-O-galactoside (M3G). The binding of SPI causes a considerable modification in its secondary structure, leading to a decrease in the β-sheet format and an increase in the α-helix, β-turn, and random coil structures. When heated to 45 °C, the combination effect of SPI and M3G was the most significant, thus improving blueberry ANs' stability. The interaction between SPI and M3G predominantly occurs via hydrophobic interactions. Furthermore, molecular dynamics simulations utilized the AMBER 18 protein force field and demonstrated that hydrogen bonds played a role as a source of force. Notably, pre-SPI exhibited superior anthocyanin wrapping capabilities to natural SPI, mitigating external environmental damage resulting from pocket tightening. These findings offer a deeper understanding of how proteins and ANs interact.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


