Photocatalyst/metal-free sequential C–N/C–S bond formation: Synthesis of S-arylisothioureas via photoinduced EDA complex activation
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
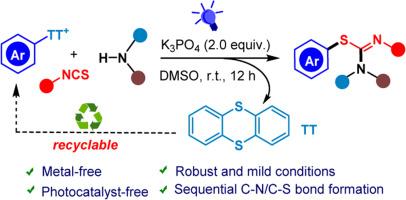
A photocatalyst-free visible-light-promoted three-component reaction of thianthrenium salts, isothiocyanates, and amines is presented, which affords a rapid and efficient approach to S-arylisothioureas under mild conditions. This developed method exhibits the advantages of readily available raw materials, broad substrate scope, good functional tolerance, and operational simplicity. It is worth mentioning that the byproduct thianthrene can be recycled in quantity, ultimately maximizing the atomic economy of the reaction and avoiding chemical waste. Mechanism investigations support the strategy involving a photoinduced EDA complex.
光催化剂/无金属顺序 C-N/C-S 键形成:通过光诱导 EDA 复合物活化合成 S-芳基异硫脲类化合物
本文介绍了一种无光催化剂可见光促进的噻吩鎓盐、异硫氰酸盐和胺的三组分反应,它提供了一种在温和条件下快速高效地制备 S-芳基异硫脲的方法。该方法具有原料易得、底物范围广、功能耐受性好和操作简单等优点。值得一提的是,副产品噻蒽可以大量回收利用,最终最大限度地提高了反应的原子经济性,避免了化学废物的产生。机理研究支持涉及光诱导 EDA 复合物的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


