Compatibility study of topical 0.25% hypericin (HyBryteTM) application in subjects with mycosis fungoides: Results of the HPN-CTCL-02 study
Abstract
Background
HyBryteTM is a photodynamic therapy of topical hypericin that has recently been shown to be safe and efficacious in early stage cutaneous T-cell lymphoma (CTCL). However, its efficacy, absorption, and effect on heart function parameters in patients who require greater HyBryteTM exposure is unknown.
Objectives
The primary objectives in this study were to assess hypericin blood levels using a validated detection method with a cut-off value of 0.05 ng/mL and to determine if topical HyBryteTM induces any electrocardiogram (EKG) changes during 8 weeks of treatment. A secondary endpoint of this study was to assess the effectiveness of HyBryteTM in this patient population as well as assessing a different additional light device than the one used in the Phase 3 HPN-CTCL-01/fluorescent light activated synthetic hypericin trial also entitled “A phase 3 multicenter randomised placebo-controlled study to determine the efficacy of topical hypericin and light irradiation for the treatment of cutaneous T-cell lymphoma”.
Methods
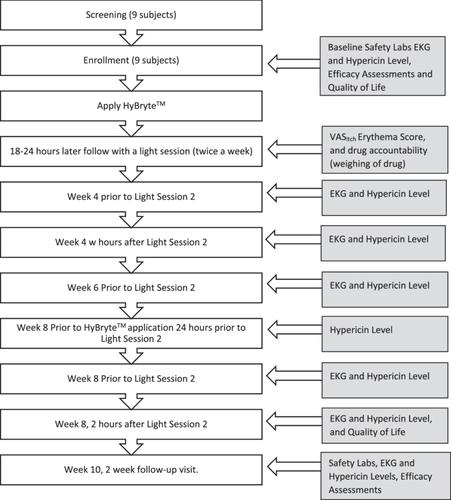
A confirmatory, prospective, open-label, single-centre, interventional study focused on stage IB and IIA mycosis fungoides with more than 10% of their body surface areas involved was performed.
Results
Hypericin concentration in K2EDTA whole blood samples collected before and after light activation at Weeks 4, 6 and 8 showed an average blood concentration of 0.13 ng/mL and achieved steady state by Week 4. EKGs were examined for clinical changes at each study visit, including changes in QT intervals and correction of heart rates. No significant clinical changes in EKGs were observed.
Conclusions
Hypericin does not appear to be significantly absorbed through the skin nor cause significant cardiac changes overall or prolong the QT interval when applied topically. A larger study is necessary to clearly define these results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


