IDH inhibition in gliomas: from preclinical models to clinical trials
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
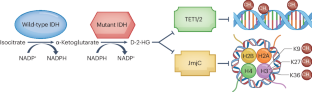
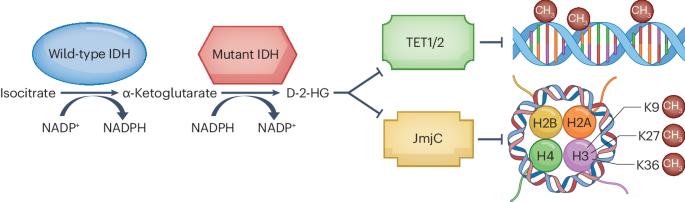
Gliomas are the most common malignant primary brain tumours in adults and cannot usually be cured with standard cancer treatments. Gliomas show intratumoural and intertumoural heterogeneity at the histological and molecular levels, and they frequently contain mutations in the isocitrate dehydrogenase 1 (IDH1) or IDH2 gene. IDH-mutant adult-type diffuse gliomas are subdivided into grade 2, 3 or 4 IDH-mutant astrocytomas and grade 2 or 3 IDH-mutant, 1p19q-codeleted oligodendrogliomas. The product of the mutated IDH genes, d-2-hydroxyglutarate (D-2-HG), induces global DNA hypermethylation and interferes with immunity, leading to stimulation of tumour growth. Selective inhibitors of mutant IDH, such as ivosidenib and vorasidenib, have been shown to reduce D-2-HG levels and induce cellular differentiation in preclinical models and to induce MRI-detectable responses in early clinical trials. The phase III INDIGO trial has demonstrated superiority of vorasidenib, a brain-penetrant pan-mutant IDH inhibitor, over placebo in people with non-enhancing grade 2 IDH-mutant gliomas following surgery. In this Review, we describe the pathway of development of IDH inhibitors in IDH-mutant low-grade gliomas from preclinical models to clinical trials. We discuss the practice-changing implications of the INDIGO trial and consider new avenues of investigation in the field of IDH-mutant gliomas. Gliomas are the most common malignant primary brain tumours in adults, and they frequently contain mutations in the isocitrate dehydrogenase 1 (IDH1) or IDH2 gene. Small-molecule inhibitors of mutant IDH are emerging as a new therapeutic strategy for IDH-mutant cancers, and this Review charts their pathway of development for IDH-mutant gliomas.
胶质瘤中的 IDH 抑制:从临床前模型到临床试验
胶质瘤是成人最常见的恶性原发性脑肿瘤,通常无法通过标准的癌症治疗方法治愈。胶质瘤在组织学和分子水平上表现出瘤内和瘤间异质性,而且经常含有异柠檬酸脱氢酶 1(IDH1)或 IDH2 基因突变。IDH突变的成人型弥漫性胶质瘤又分为2、3或4级IDH突变星形细胞瘤和2或3级IDH突变、1p19q编码缺失少突胶质瘤。突变 IDH 基因的产物--d-2-羟基戊二酸(D-2-HG)会诱导 DNA 整体超甲基化并干扰免疫,从而刺激肿瘤生长。突变型IDH的选择性抑制剂,如ivosidenib和vorasidenib,已被证明能降低D-2-HG水平,诱导临床前模型中的细胞分化,并在早期临床试验中诱导MRI可检测到的反应。INDIGO III 期试验表明,对于术后无增强的 2 级 IDH 突变胶质瘤患者,脑穿透性泛突变 IDH 抑制剂 vorasidenib 的疗效优于安慰剂。在本综述中,我们描述了IDH抑制剂在IDH突变低级别胶质瘤中从临床前模型到临床试验的发展路径。我们讨论了 INDIGO 试验对实践的影响,并探讨了 IDH 突变胶质瘤领域的新研究途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


