Homogeneous multi-payload antibody–drug conjugates
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Many systemic cancer chemotherapies comprise a combination of drugs, yet all clinically used antibody–drug conjugates (ADCs) contain a single-drug payload. These combination regimens improve treatment outcomes by producing synergistic anticancer effects and slowing the development of drug-resistant cell populations. In an attempt to replicate these regimens and improve the efficacy of targeted therapy, the field of ADCs has moved towards developing techniques that allow for multiple unique payloads to be attached to a single antibody molecule with high homogeneity. However, the methods for generating such constructs—homogeneous multi-payload ADCs—are both numerous and complex owing to the plethora of reactive functional groups that make up the surface of an antibody. Here, by summarizing and comparing the methods of both single- and multi-payload ADC generation and their key preclinical and clinical results, we provide a timely overview of this relatively new area of research. The methods discussed range from branched linker installation to the incorporation of unnatural amino acids, with a generalized comparison tool of the most promising modification strategies also provided. Finally, the successes and challenges of this rapidly growing field are critically evaluated, and from this, future areas of research and development are proposed. Multi-payload antibody–drug conjugates (ADCs) are an emerging class of targeted therapeutics. Comprising a monoclonal antibody with multiple unique payloads attached, these constructs have the potential to produce synergistic anticancer effects with reduced therapeutic resistance. In this Review, methods for generating multi-payload ADCs are discussed, highlighting some key preclinical results.
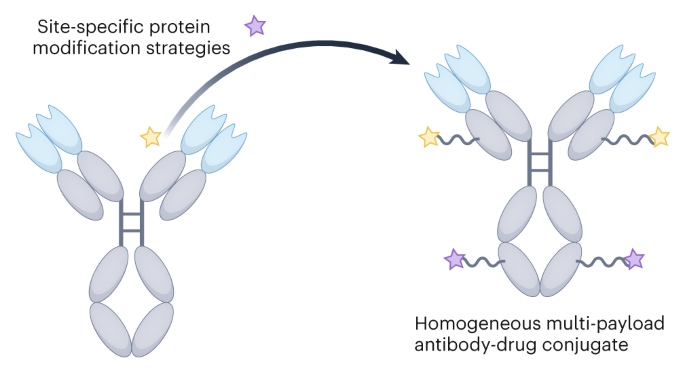
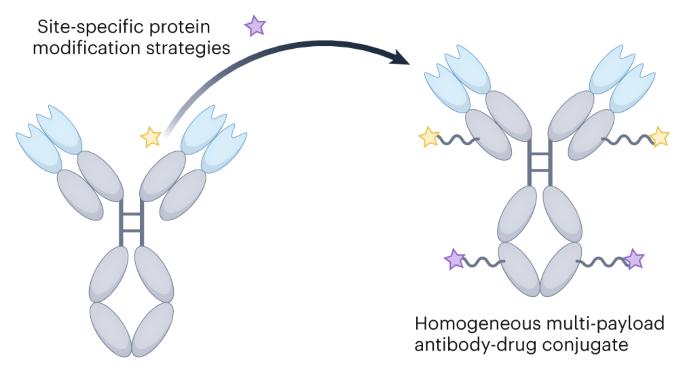
均相多负载抗体-药物共轭物
许多全身性癌症化疗药物都是由多种药物组合而成,但临床上使用的所有抗体药物共轭物(ADC)都含有单药有效载荷。这些联合疗法可产生协同抗癌效果,并减缓耐药细胞群的发展,从而改善治疗效果。为了复制这些治疗方案并提高靶向治疗的疗效,ADC 领域已转向开发可将多种独特有效载荷连接到单一抗体分子上且具有高度同质性的技术。然而,由于抗体表面存在大量反应性官能团,因此生成这种构建体--同质多载荷 ADC 的方法既多又复杂。在这里,我们总结并比较了单载量和多载量 ADC 的生成方法及其关键的临床前和临床结果,从而及时概述了这一相对较新的研究领域。讨论的方法包括从支链连接体的安装到非天然氨基酸的加入,同时还提供了最有前途的修饰策略的通用比较工具。最后,我们对这一迅速发展的领域所取得的成功和面临的挑战进行了批判性评估,并由此提出了未来的研究和发展领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


