Supramolecular trapping of a cationic all-metal σ-aromatic {Bi4} ring
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aromaticity in organic molecules is well defined, but its role in metal-only rings remains controversial. Here we introduce a supramolecular stabilization approach of a cationic {Bi4} rhomboid within the symmetric charge sphere of two bowl-shaped dianionic calix[4]pyrrolato indinates. Crystallographic and spectroscopic characterization, quantum chemical analysis and magnetically induced ring currents indicate σ-aromaticity in the formally tetracationic 16-valence electron [Bi4]4+ ring. Computational screening for other p-block elements identifies the planar rhomboid as the globally preferred structure for 16-valence electron four-atomic clusters. The aromatic [Bi4]4+ is isoelectronic to the [Al4]4−, a motif previously observed as antiaromatic in Li3[Al4]− in the gas phase. Thus, subtle factors such as charge isotropy seem to decide over aromaticity or antiaromaticity, advising for caution in debates based on the Hückel model—a concept valid for second-row elements but less deterministic for the heavier congeners. The synthesis of cationic all-metal aromatic systems without covalent functionalization remains an underexplored area in chemistry. Now a tetracationic [Bi4]4+ featuring all-metal σ-aromaticity has been stabilized through a supramolecular approach relying on dianionic calix[4]pyrrolato indiumbromide shells. This planar rhomboid represents the global minimum for 16 valence electron systems.
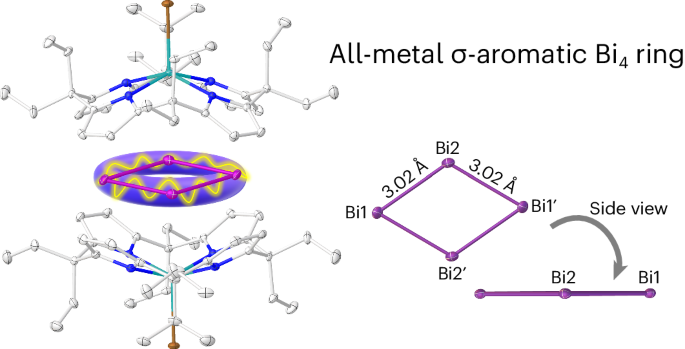
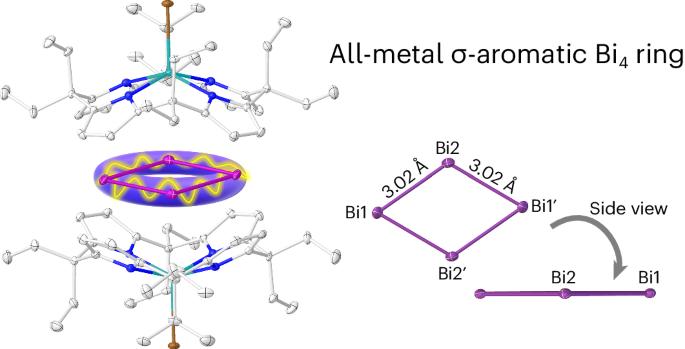
阳离子全金属σ芳香族{Bi4}环的超分子捕获
有机分子中的芳香性已有明确定义,但其在纯金属环中的作用仍存在争议。在这里,我们介绍了一种超分子稳定方法,即在两个碗状二阴离子钙[4]吡咯烷吲哚的对称电荷球内稳定阳离子{Bi4}斜方体。晶体学和光谱学表征、量子化学分析以及磁感应环电流表明,形式上为四阳离子的 16 价电子 [Bi4]4+ 环具有σ芳香性。通过对其他 p 块元素的计算筛选,确定平面斜方体是 16 价电子四原子团簇的全球首选结构。芳香[Bi4]4+与[Al4]4-是等电子的,而之前在气相中观察到的 Li3[Al4]- 的芳香[Bi4]4+与[Al4]4-是反芳香的。因此,电荷各向同性等微妙因素似乎决定了芳香性或反芳香性,建议在基于胡克尔模型的辩论中谨慎从事--这一概念对第二排元素有效,但对较重的同系物则不那么确定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


