mRNA-LNP prime boost evolves precursors toward VRC01-like broadly neutralizing antibodies in preclinical humanized mouse models
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
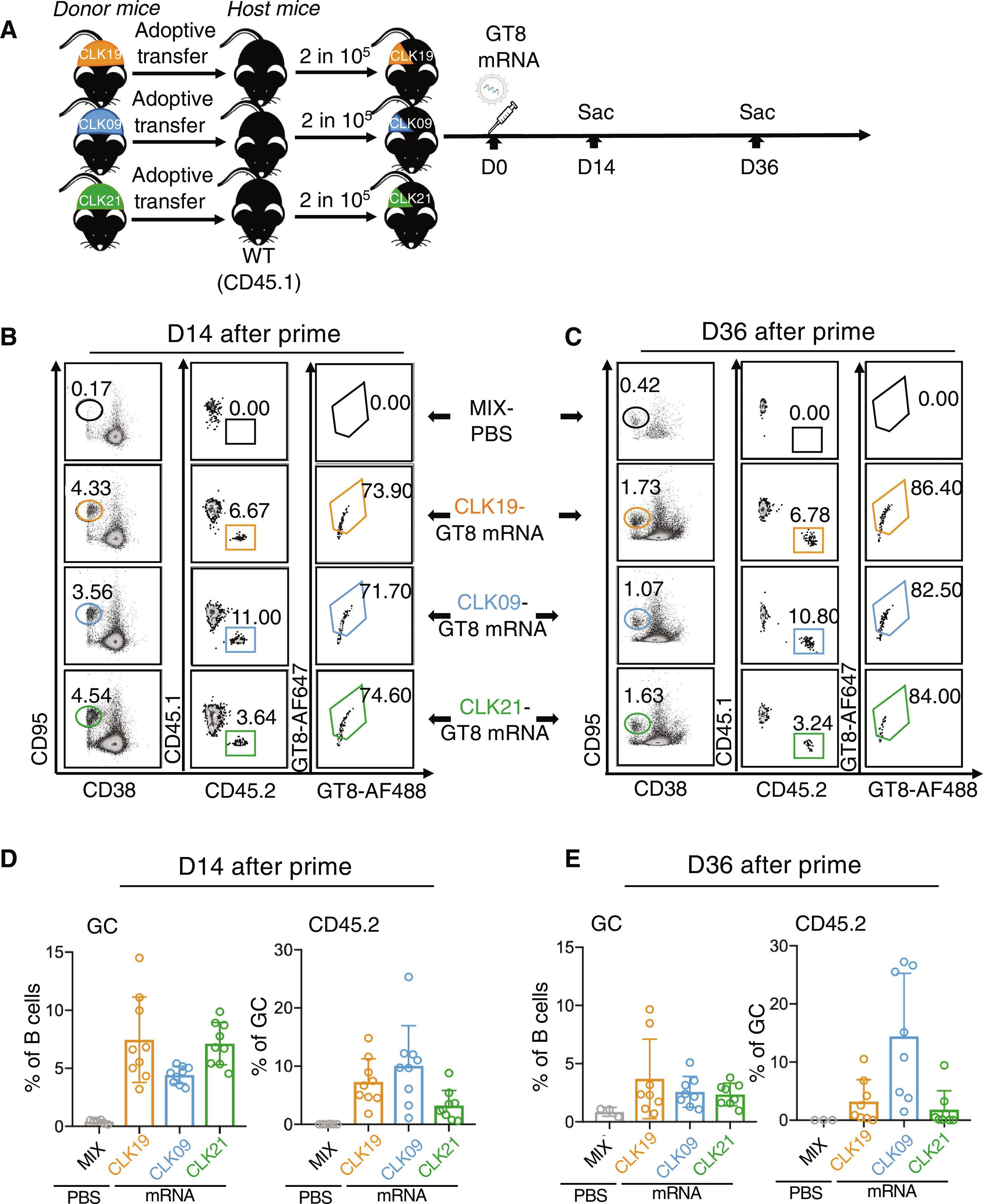
Germline-targeting (GT) protein immunogens to induce VRC01-class broadly neutralizing antibodies (bnAbs) to the CD4-binding site of the HIV envelope (Env) have shown promise in clinical trials. Here, we preclinically validated a lipid nanoparticle–encapsulated nucleoside mRNA (mRNA-LNP) encoding eOD-GT8 60mer as a soluble self-assembling nanoparticle in mouse models. In a model with three humanized B cell lineages bearing distinct VRC01-precursor B cell receptors (BCRs) with similar affinities for eOD-GT8, all lineages could be simultaneously primed and undergo diversification and affinity maturation without exclusionary competition. Boosts drove precursor B cell participation in germinal centers; the accumulation of somatic hypermutations, including in key VRC01-class positions; and affinity maturation to boost and native-like antigens in two of the three precursor lineages. We have preclinically validated a prime-boost regimen of soluble self-assembling nanoparticles encoded by mRNA-LNP, demonstrating that multiple lineages can be primed, boosted, and diversified along the bnAb pathway.
在临床前人源化小鼠模型中,mRNA-LNP 推动前体向类似于 VRC01 的广谱中和抗体进化
胚系靶向(GT)蛋白免疫原可诱导针对艾滋病病毒包膜(Env)CD4结合位点的VRC01级广谱中和抗体(bnAbs),已在临床试验中显示出良好的前景。在这里,我们在小鼠模型中对编码 eOD-GT8 60mer 的脂质纳米颗粒封装核苷 mRNA(mRNA-LNP)作为可溶性自组装纳米颗粒进行了临床前验证。在具有三个人源化 B 细胞系的模型中,这些细胞系都带有不同的 VRC01 前体 B 细胞受体(BCR),它们对 eOD-GT8 具有相似的亲和力。增殖推动了前体 B 细胞参与生殖中心;体细胞高突变的积累,包括在关键的 VRC01 类位置;以及三个前体系中两个系对增殖抗原和类原生抗原的亲和性成熟。我们在临床前验证了由 mRNA-LNP 编码的可溶性自组装纳米粒子的启动-启动方案,证明了多系细胞可沿着 bnAb 途径启动、启动和多样化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


