Zinc–iodine redox reaction enables direct brine valorization with efficient high-water-recovery desalination
引用次数: 0
Abstract
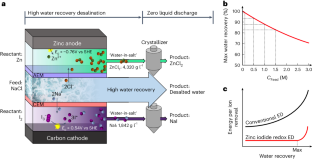
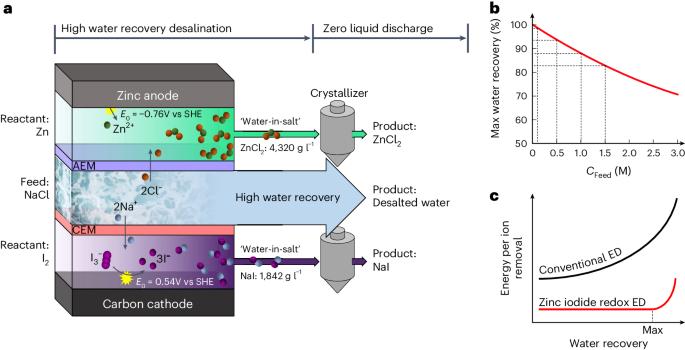
Current desalination methods, with high energy/cost demands and large volumes of brine discharged to the environment, are not sustainable. Here we propose a sustainable electrodialysis that enables direct brine valorization with efficient high-water-recovery desalination via zinc–iodine redox reactions. In a single process comprising electrodialysis and two chemical reactions in brine streams, we achieve seawater desalination with a remarkable water recovery of 90.09% without compromising other metrics (salt-removal ratio <98.29%, electric energy consumption of <2.18 kWh m−3). Such performance advantage is attributable to (1) high solubility of zinc–iodine-based ‘water-in-salt’ electrolytes mitigating the osmotic pressure, achieving high water recovery even for high concentration feed water (98–82% for 0.1–1.5 M) with minimal energy burdens, (2) zinc–iodine redox potential lowering electric energy demand and (3) electroconvection in the overlimiting regime enhancing desalination speed. Also, profitable ZnCl2/NaI are electrosynthesized in brine, enabling direct valorization of desalination brines. An electrodialysis desalination process based on zinc–iodine redox reactions enables brine valorization with high efficiency of water recovery.
锌碘氧化还原反应实现了盐水的直接价值化和高效的高水回收率海水淡化
目前的海水淡化方法对能源/成本的要求很高,大量的盐水被排放到环境中,因此是不可持续的。在此,我们提出了一种可持续的电渗析方法,通过锌碘氧化还原反应实现盐水的直接价值化和高效的高水回收率海水淡化。在由电渗析和盐水流中的两种化学反应组成的单一工艺中,我们实现了海水淡化,水回收率高达 90.09%,且不影响其他指标(盐去除率为 98.29%,电能消耗为 2.18 kWh m-3)。这种性能优势归功于:(1)锌碘基 "盐中水 "电解质的高溶解度减轻了渗透压,即使是高浓度进水(0.1-1.5 M 时为 98-82%)也能实现高水回收率,且能耗极低;(2)锌碘氧化还原电位降低了电能需求;(3)超限状态下的电对流提高了脱盐速度。此外,在盐水中电合成 ZnCl2/NaI 还能带来利润,从而实现海水淡化盐水的直接增值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


