CircST6GAL1 knockdown alleviates pulmonary arterial hypertension by regulating miR-509-5p/multiple C2 and transmembrane domain containing 2 axis
Abstract
Background
Hypertension is a main contributing factor of cardiovascular diseases; deregulated circular RNAs are involved in the pathogenesis of pulmonary arterial hypertension (PAH). Herein, we evaluated the function and mechanism of circST6GAL1 in PAH process.
Methods
Human pulmonary artery smooth muscle cells (HPASMCs) were cultured in hypoxic environment for functional analysis. The cell counting kit-8, 5-ethynyl-2′-deoxyuridine, wound healing, and flow cytometry assays were used to investigate cell proliferation, migration, and apoptosis. qRT-PCR and Western blotting analyses were used for level measurement of genes and proteins. The binding between miR-509-5p and circST6GAL1 or multiple C2 and transmembrane domain containing 2 (MCTP2) was analyzed by dual-luciferase reporter, RNA immunoprecipitation, and pull-down assays. The monocrotaline (MCT)-induced PAH mouse models were established for in vivo assay.
Results
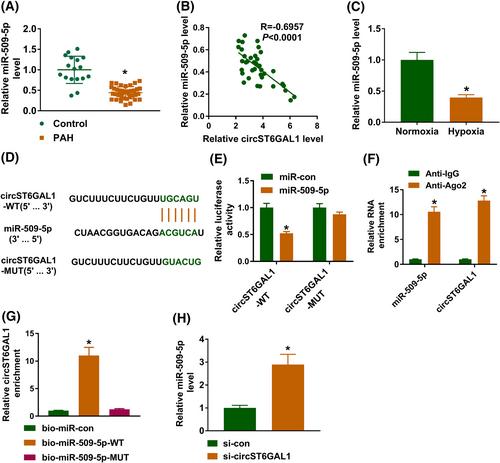
CircST6GAL1 was highly expressed in PAH patients and hypoxia-induced HPASMCs. Functionally, circST6GAL1 deficiency reversed hypoxia-induced proliferation and migration, as well as apoptosis arrest in HPASMCs. Mechanistically, circST6GAL1 directly targeted miR-509-5p, and MCTP2 was a target of miR-509-5p. Rescue assays showed that the regulatory effects of circST6GAL1 deficiency on hypoxia-induced HPASMCs were abolished. Moreover, forced expression of miR-509-5p suppressed HPASMC proliferation and migration and induced cell apoptosis under hypoxia stimulation, while these effects were abolished by MCTP2 overexpression. Moreover, circST6GAL1 silencing improved MCT-induced pulmonary vascular remodeling and PAH.
Conclusion
CircST6GAL1 deficiency reversed hypoxia-induced proliferation and migration, as well as apoptosis arrest in HPASMCs, and alleviated pulmonary vascular remodeling in MCT-induced PAH mouse models through the miR-509-5p/MCTP2 axis, indicating a potential therapeutic target for PAH.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


