Targeting TGFβ-activated kinase-1 activation in microglia reduces CAR T immune effector cell-associated neurotoxicity syndrome
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
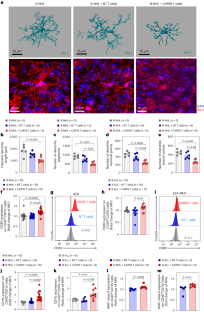
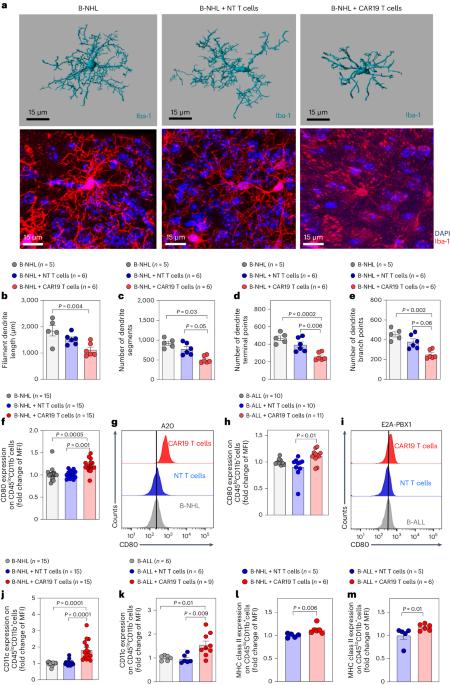
Cancer immunotherapy with chimeric antigen receptor (CAR) T cells can cause immune effector cell-associated neurotoxicity syndrome (ICANS). However, the molecular mechanisms leading to ICANS are not well understood. Here we examined the role of microglia using mouse models and cohorts of individuals with ICANS. CD19-directed CAR (CAR19) T cell transfer in B cell lymphoma-bearing mice caused microglia activation and neurocognitive deficits. The TGFβ-activated kinase-1 (TAK1)–NF-κB–p38 MAPK pathway was activated in microglia after CAR19 T cell transfer. Pharmacological TAK1 inhibition or genetic Tak1 deletion in microglia using Cx3cr1CreER:Tak1fl/fl mice resulted in reduced microglia activation and improved neurocognitive activity. TAK1 inhibition allowed for potent CAR19-induced antilymphoma effects. Individuals with ICANS exhibited microglia activation in vivo when studied by translocator protein positron emission tomography, and imaging mass cytometry revealed a shift from resting to activated microglia. In summary, we prove a role for microglia in ICANS pathophysiology, identify the TAK1–NF-κB–p38 MAPK axis as a pathogenic signaling pathway and provide a rationale to test TAK1 inhibition in a clinical trial for ICANS prevention after CAR19 T cell-based cancer immunotherapy. Zeiser and colleagues show that CAR T cell therapy results in upregulation of the TGFβ-activated kinase-1 (TAK1)–NF-κB–p38 MAPK pathway in microglia, causing neurocognitive defects, and find that TAK1 inhibition can reduce immune effector cell-associated neurotoxicity syndrome.
靶向激活小胶质细胞中的 TGFβ 激活激酶-1 可减轻 CAR T 免疫效应细胞相关神经毒性综合征。
使用嵌合抗原受体(CAR)T细胞进行癌症免疫疗法会导致免疫效应细胞相关神经毒性综合征(ICANS)。然而,导致 ICANS 的分子机制尚不十分清楚。在这里,我们利用小鼠模型和 ICANS 患者队列研究了小胶质细胞的作用。CD19定向CAR(CAR19)T细胞转移到B细胞淋巴瘤小鼠体内会导致小胶质细胞活化和神经认知障碍。CAR19 T细胞转移后,TGFβ激活的激酶-1(TAK1)-NF-κB-p38 MAPK通路在小胶质细胞中被激活。利用 Cx3cr1CreER:Tak1fl/fl 小鼠对小胶质细胞中的 TAK1 进行药理抑制或基因Tak1 基因缺失,可减少小胶质细胞的活化并改善神经认知活动。抑制 TAK1 可实现 CAR19 诱导的强效抗淋巴瘤作用。通过转运体蛋白正电子发射断层扫描研究,患有 ICANS 的个体在体内表现出小胶质细胞活化,成像质控细胞仪显示了小胶质细胞从静止到活化的转变。总之,我们证明了小胶质细胞在 ICANS 病理生理学中的作用,确定了 TAK1-NF-κB-p38 MAPK 轴为致病信号通路,并为在基于 CAR19 T 细胞的癌症免疫疗法后的 ICANS 预防临床试验中测试 TAK1 抑制作用提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


