Recent advancements in cartilage tissue engineering innovation and translation
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
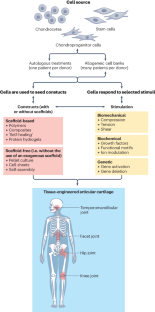
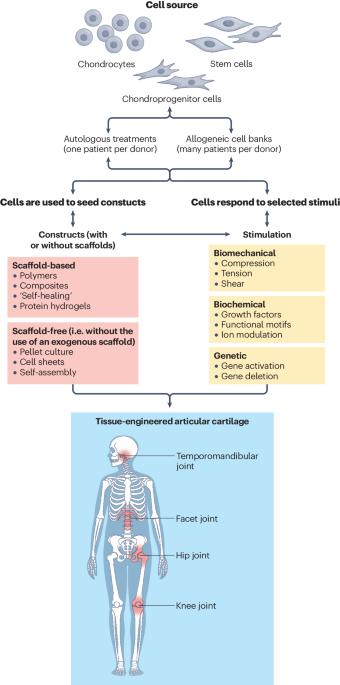
Articular cartilage was expected to be one of the first successfully engineered tissues, but today, cartilage repair products are few and they exhibit considerable limitations. For example, of the cell-based products that are available globally, only one is marketed for non-knee indications, none are indicated for severe osteoarthritis or rheumatoid arthritis, and only one is approved for marketing in the USA. However, advances in cartilage tissue engineering might now finally lead to the development of new cartilage repair products. To understand the potential in this field, it helps to consider the current landscape of tissue-engineered products for articular cartilage repair and particularly cell-based therapies. Advances relating to cell sources, bioactive stimuli and scaffold or scaffold-free approaches should now contribute to progress in therapeutic development. Engineering for an inflammatory environment is required because of the need for implants to withstand immune challenge within joints affected by osteoarthritis or rheumatoid arthritis. Bringing additional cartilage repair products to the market will require an understanding of the translational vector for their commercialization. Advances thus far can facilitate the future translation of engineered cartilage products to benefit the millions of patients who suffer from cartilage injuries and arthritides. In this Review, the current landscape of tissue engineering for repair of articular cartilage is discussed, with reference to advances in cell sources, bioactive stimuli and the use of scaffolds, and with consideration of the challenges that result from the inflammatory articular environments in osteoarthritis and rheumatoid arthritis.
软骨组织工程创新和转化的最新进展
关节软骨有望成为首批成功的工程组织之一,但目前软骨修复产品很少,而且有相当大的局限性。例如,在全球上市的基于细胞的产品中,只有一种用于非膝关节适应症,没有一种用于严重的骨关节炎或类风湿性关节炎,只有一种获准在美国上市。不过,软骨组织工程学的进步现在可能最终导致开发新的软骨修复产品。要了解这一领域的潜力,不妨先了解一下目前用于关节软骨修复的组织工程产品,尤其是基于细胞的疗法。细胞来源、生物活性刺激物、支架或无支架方法等方面的进步应有助于治疗开发的进展。由于受骨关节炎或类风湿性关节炎影响的关节需要植入物来抵御免疫挑战,因此需要针对炎症环境进行工程设计。要将更多软骨修复产品推向市场,就必须了解其商业化的转化载体。迄今为止取得的进展可以促进工程软骨产品的未来转化,使数百万软骨损伤和关节炎患者受益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


