Shikonin and chitosan-silver nanoparticles synergize against triple-negative breast cancer through RIPK3-triggered necroptotic immunogenic cell death
Abstract
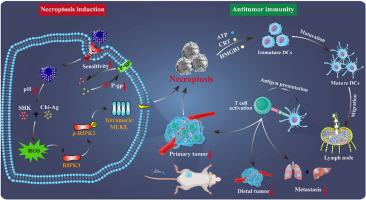
Necroptotic immunogenic cell death (ICD) can activate the human immune system to treat the metastasis and recurrence of triple-negative breast cancer (TNBC). However, developing the necroptotic inducer and precisely delivering it to the tumor site is the key issue. Herein, we reported that the combination of shikonin (SHK) and chitosan silver nanoparticles (Chi-Ag NPs) effectively induced ICD by triggering necroptosis in 4T1 cells. Moreover, to address the lack of selectivity of drugs for in vivo application, we developed an MUC1 aptamer-targeted nanocomplex (MUC1@Chi-Ag@CPB@SHK, abbreviated as MUC1@ACS) for co-delivering SHK and Chi-Ag NPs. The accumulation of MUC1@ACS NPs at the tumor site showed a 6.02-fold increase compared to the free drug. Subsequently, upon reaching the tumor site, the acid-responsive release of SHK and Chi-Ag NPs from MUC1@ACS NPs cooperatively induced necroptosis in tumor cells by upregulating the expression of RIPK3, p-RIPK3, and tetrameric MLKL, thereby effectively triggering ICD. The sequential maturation of dendritic cells (DCs) subsequently enhanced the infiltration of CD8+ and CD4+ T cells in tumors, while inhibiting regulatory T cells (Treg cells), resulting in the effective treatment of primary and distal tumor growth and the inhibition of TNBC metastasis. This work highlights the importance of nanoparticles in mediating drug interactions during necroptotic ICD.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|---|---|
| 麦克林 |
Sulfo-SMCC
|
¥78.00~¥131705.00 |
| Sigma |
Chitosan
|
¥24.00~¥24532.00 |
| 上海源叶 |
Z-VAD-FMK
|
¥425.00~¥12500.00 |
| 上海源叶 |
GSK-872
|
¥131.00~¥9870.00 |
| 上海源叶 |
GSK-872
|
|
| 上海源叶 |
Z-VAD-FMK
|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


