Bacteriocin diversity, function, discovery and application as antimicrobials
IF 69.2
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Bacteriocins are potent antimicrobial peptides that are produced by bacteria. Since their discovery almost a century ago, diverse peptides have been discovered and described, and some are currently used as commercial food preservatives. Many bacteriocins exhibit extensively post-translationally modified structures encoded on complex gene clusters, whereas others have simple linear structures. The molecular structures, mechanisms of action and resistance have been determined for a number of bacteriocins, but most remain incompletely characterized. These gene-encoded peptides are amenable to bioengineering strategies and heterologous expression, enabling metagenomic mining and modification of novel antimicrobials. The ongoing global antimicrobial resistance crisis demands that novel therapeutics be developed to combat infectious pathogens. New compounds that are target-specific and compatible with the resident microbiota would be valuable alternatives to current antimicrobials. As bacteriocins can be broad or narrow spectrum in nature, they are promising tools for this purpose. However, few bacteriocins have gone beyond preclinical trials and none is currently used therapeutically in humans. In this Review, we explore the broad diversity in bacteriocin structure and function, describe identification and optimization methods and discuss the reasons behind the lack of translation beyond the laboratory of these potentially valuable antimicrobials. In this Review, Sugrue, Ross and Hill explore recent developments in bacteriocin research, including new discoveries and bioengineering approaches for improved activity, and discuss their application in microbiome modulation and clinical potential.
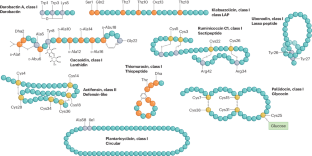
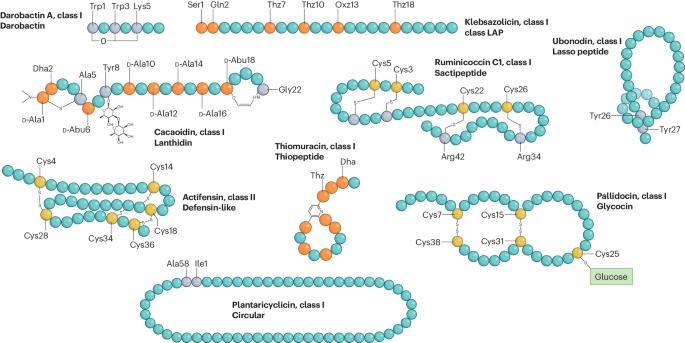
细菌素的多样性、功能、发现以及作为抗菌剂的应用
细菌素是由细菌产生的强效抗菌肽。自近一个世纪前被发现以来,人们发现并描述了多种肽,其中一些目前被用作商业食品防腐剂。许多细菌素的结构在复杂的基因簇编码下经过广泛的翻译后修饰,而其他细菌素则具有简单的线性结构。一些细菌素的分子结构、作用机制和抗药性已经确定,但大多数细菌素的特征还不完全清楚。这些基因编码的肽适合生物工程策略和异源表达,可用于元基因组挖掘和改造新型抗菌素。当前全球抗菌药耐药性危机要求开发新型疗法来对抗传染性病原体。具有靶向特异性并与常住微生物群相容的新化合物将成为当前抗菌剂的重要替代品。由于细菌素可以是广谱或窄谱的,因此它们是实现这一目的的有前途的工具。然而,很少有细菌素能超越临床前试验,目前也没有一种细菌素能用于人类治疗。在这篇综述中,我们探讨了细菌素结构和功能的广泛多样性,介绍了鉴定和优化方法,并讨论了这些具有潜在价值的抗菌素未能应用于实验室以外领域的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Microbiology
生物-微生物学
CiteScore
74.00
自引率
0.50%
发文量
149
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Microbiology, our goal is to become the leading source of reviews and commentaries for the scientific community we cater to. We are dedicated to publishing articles that are not only authoritative but also easily accessible, supplementing them with clear and concise figures, tables, and other visual aids. Our objective is to offer an unparalleled service to authors, referees, and readers, and we continuously strive to maximize the usefulness and impact of each article we publish. With a focus on Reviews, Perspectives, and Comments spanning the entire field of microbiology, our wide scope ensures that the work we feature reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


