Inflammation-associated intramyocellular lipid alterations in human pancreatic cancer cachexia
Abstract
Background
Cancer cachexia is a multifactorial metabolic syndrome characterized by systemic inflammation and ongoing skeletal muscle loss resulting in weakness, poor quality of life, and decreased survival. Whereas lipid accumulation in skeletal muscle is associated with cancer cachexia as well as the prognosis of cancer patients, surprisingly little is known about the nature of the lipids that accumulate in the muscle during cachexia, and whether this is related to inflammation. We aimed to identify the types and distributions of intramyocellular lipids in patients with and without cancer cachexia.
Methods
Rectus abdominis muscle biopsies were collected during surgery of patients with pancreatic ductal adenocarcinoma (n = 10 without cachexia, n = 20 cachectic without inflammation (CRP < 10 mg/L), n = 10 cachectic with inflammation (CRP ≥ 10 mg/L). L3-CT scans were analysed to assess body composition based on validated thresholds in Hounsfield units (HU). Muscle sections were stained with Oil-Red O and H&E to assess general lipid accumulation and atrophy. Untargeted lipidomic analyses were performed on laser-microdissected myotubes using LC–MS/MS. The spatial distribution of intramyocellular lipids with differential abundance between groups was visualized by mass-spectrometry imaging. Genes coding for inflammation markers and enzymes involved in de novo ceramide synthesis were studied by qPCR.
Results
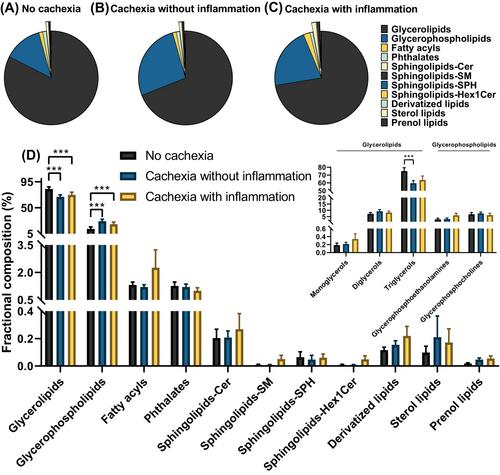
Muscle radiation attenuation was lower in cachectic patients with inflammation (median 24.3 [18.6–30.8] HU) as compared with those without inflammation (34.2 [29.3–38.7] HU, P = 0.033) or no cachexia (37.4 [33.9–42.9] HU, P = 0.012). Accordingly, intramyocellular lipid content was lower in non-cachectic patients (1.9 [1.6–2.1]%) as compared with those with cachexia with inflammation (5.5 [4.5–7.3]%, P = 0.002) or without inflammation (4.8 [2.6–6.0]%, P = 0.017). Intramyocellular lipid accumulation was associated with both local IL-6 mRNA levels (rs = 0.57, P = 0.015) and systemic CRP levels (rs = 0.49, P = 0.024). Compared with non-cachectic subjects, cachectic patients had a higher relative abundance of intramyocellular glycerophospholipids and a lower relative abundance of glycerolipids. Furthermore, increases in several intramyocellular lipids such as SM(d36:1), PC(34:1), and TG(48:1) were found in cachectic patients with inflammation and correlated with specific cachexia features. Altered intramyocellular lipid species such as PC(34:1), LPC(18:2), and TG(48:1) showed an uneven distribution in muscle sections of cachectic and non-cachectic patients, with areas featuring abundance of these lipids next to areas almost devoid of them.
Conclusions
Intramyocellular lipid accumulation in patients with cachexia is associated with both local and systemic inflammation, and characterized by changes in defined lipid species such as glycerolipids and glycerophospholipids.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


