BF3·Et2O-assisted synthesis of sulfinylated spiro[5.5]trienones from biaryl ynones†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Sulfinyls are valuable structural moieties used for developing synthetically new pharmaceuticals and agrochemicals. Herein, we disclose a straightforward synthesis of sulfinylated spiro[5.5]trienones proceeding via an unprecedented BF3·Et2O-promoted spirocyclization of biaryl ynones. The availability of relatively inexpensive BF3·Et2O to carry out transformations on a bulk scale along with its further application towards the synthesis of dibenzocyclohepten-5-ones delivers a unique opportunity to deploy it in various synthetic directions.
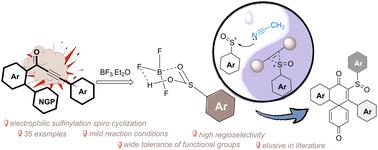
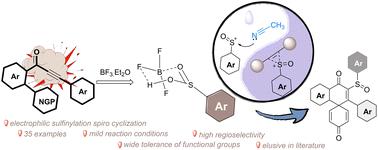
BF3-Et2O 辅助从双芳基炔酮合成亚砜基螺[5.5]三烯酮。
亚磺酰基是用于开发合成新药和农用化学品的重要结构分子。在此,我们公开了一种直接合成亚磺酰基螺[5.5]三烯酮的方法,该方法是通过前所未有的 BF3-Et2O 促进双芳基炔酮的螺环化反应进行的。相对廉价的 BF3-Et2O 可用于进行大规模转化,它还可进一步用于合成二苯并环庚烯-5-酮,这为将其应用于各种合成方向提供了独特的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


