The Role of Soluble Urokinase Plasminogen Activator Receptor (suPAR) as an Early Indicator of Mortality in Pediatric Septic Shock
Abstract
Background
Despite advancements in antibiotic therapy and resuscitation protocols, sepsis and septic shock remain major contributors to morbidity and mortality in children. We aimed to investigate the utility of soluble urokinase plasminogen activator receptor (suPAR) for the early detection of septic shock and to evaluate its accuracy in predicting mortality.
Methods
A prospective study was conducted in a tertiary pediatric emergency department (ED), enrolling patients diagnosed with the sepsis, severe sepsis, or septic shock. In addition to assessing infection biomarkers such as C-reactive protein and procalcitonin, suPAR levels were quantified upon admission using enzyme-linked immunosorbent assay. The primary outcome measure was 30-day mortality.
Results
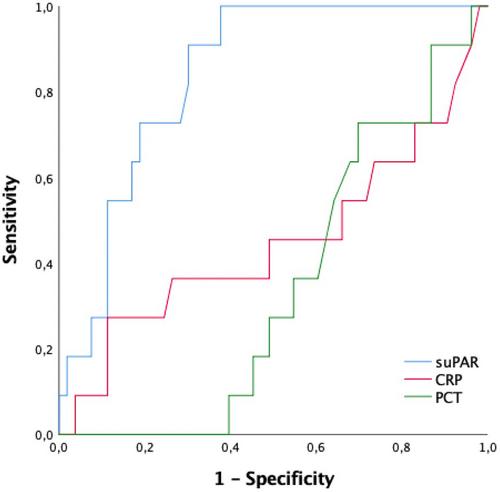
Overall 72 patients and 80 healthy children included. Plasma suPAR levels demonstrated a statistically significant elevation in the sepsis, severe sepsis, and septic shock groups compared with the control group (p < 0.001 for all). The septic shock group exhibited the highest suPAR levels upon admission, surpassing both the sepsis and severe sepsis groups (p = 0.009 and 0.042). ROC analysis underscored the promising potential of suPAR with an AUC of 0.832 for septic shock. Analysis of mortality prediction revealed significantly higher suPAR levels in nonsurvivors than survivors (9.7 ng/mL vs. 4.2 ng/mL; p < 0.001). Employing plasma suPAR levels to discriminate between mortality and survival, a threshold of ≥7.0 ng/mL demonstrated a sensitivity of 90.9% and specificity of 71.0%.
Conclusion
Plasma suPAR levels have the potential as a biomarker for predicting mortality in children with septic shock. In pediatric septic shock, the presence of plasma suPAR ≥7 ng/mL along with an underlying disease significantly increases the risk of mortality.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


