De-simplifying antiretroviral therapy from a single-tablet to a two-tablet regimen: Acceptance, patient-reported outcomes, and cost savings in a multicentre study
Abstract
Background
Antiretroviral therapy (ART), which is increasingly used by people with HIV, accounts for significant care costs, particularly because of single-tablet regimens (STRs). This study explored de-simplification to a two-tablet regimen (TTR) for cost reduction. The objectives of this study were: (1) acceptance of de-simplification, (2) patient-reported outcomes, and (3) cost savings.
Methods
All individuals on Triumeq®, Atripla® or Eviplera® in five HIV clinics in the Netherlands were eligible. Healthcare providers informed individuals of this study. After inclusion, individuals were free to de-simplify. An electronic questionnaire was sent to assess study acceptance, adherence, quality of life (SF12) and treatment satisfaction (HIVTSQ). After 3 and 12 months, questionnaires were repeated. Cost savings were calculated using Dutch drug prices.
Results
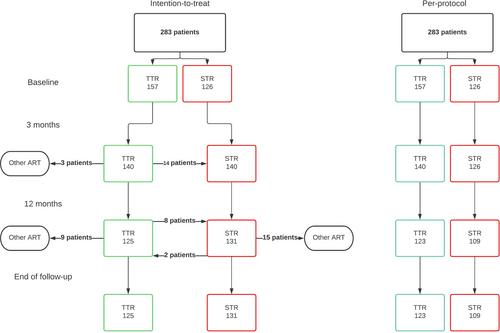
In total, 283 individuals were included, of whom 55.5% agreed to de-simplify their ART, with a large variability between treatment centres: 41.1–74.2%. Individuals who were willing to de-simplify tended to be older, had a longer history of HIV diagnosis, and used more co-medication than those who preferred to remain on an STR regimen. Patient-reported outcomes, including quality of life and treatment satisfaction, showed no significant difference between people with HIV who switched to a TTR and those who remained on an STR regimen. Furthermore, we observed a 17.8% reduction in drug costs in our cohort of people with HIV who were initially on an STR.
Conclusions
De-simplification from an STR to a TTR within the Dutch healthcare setting has been demonstrated as feasible, leads to significant cost reductions and should be discussed with every eligible person with HIV in the Netherlands.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


