Seeking Cells, Targeting Bacteria: A Cascade-Targeting Bacteria-Responsive Nanosystem for Combating Intracellular Bacterial Infections
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Intracellular bacteria pose a great challenge to antimicrobial therapy due to various physiological barriers at both cellular and bacterial levels, which impede drug penetration and intracellular targeting, thereby fostering antibiotic resistance and yielding suboptimal treatment outcomes. Herein, a cascade-target bacterial-responsive drug delivery nanosystem, MM@SPE NPs, comprising a macrophage membrane (MM) shell and a core of SPE NPs. SPE NPs consist of phenylboronic acid-grafted dendritic mesoporous silica nanoparticles (SP NPs) encapsulated with epigallocatechin-3-gallate (EGCG), a non-antibiotic antibacterial component, via pH-sensitive boronic ester bonds are introduced. Upon administration, MM@SPE NPs actively home in on infected macrophages due to the homologous targeting properties of the MM shell, which is subsequently disrupted during cellular endocytosis. Within the cellular environment, SPE NPs expose and spontaneously accumulate around intracellular bacteria through their bacteria-targeting phenylboronic acid groups. The acidic bacterial microenvironment further triggers the breakage of boronic ester bonds between SP NPs and EGCG, allowing the bacterial-responsive release of EGCG for localized intracellular antibacterial effects. The efficacy of MM@SPE NPs in precisely eliminating intracellular bacteria is validated in two rat models of intracellular bacterial infections. This cascade-targeting responsive system offers new solutions for treating intracellular bacterial infections while minimizing the risk of drug resistance.
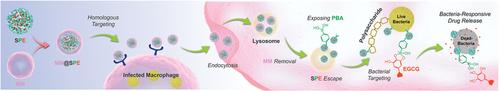
寻找细胞,锁定细菌:用于抗击细胞内细菌感染的级联靶向细菌反应纳米系统
由于细胞和细菌水平上的各种生理障碍阻碍了药物的渗透和细胞内靶向性,从而产生抗生素耐药性并导致治疗效果不理想,因此细胞内细菌给抗菌治疗带来了巨大挑战。本文提出了一种级联靶向细菌响应给药纳米系统--MM@SPE NPs,它由巨噬细胞膜(MM)外壳和SPE NPs内核组成。SPE NPs由苯基硼酸接枝树枝状介孔二氧化硅纳米颗粒(SP NPs)组成,通过pH敏感的硼酸酯键封装了非抗生素抗菌成分表没食子儿茶素-3-棓酸盐(EGCG)。给药后,由于 MM 外壳的同源靶向特性,MM@SPE NPs 会主动进入受感染的巨噬细胞,随后在细胞内吞过程中被破坏。在细胞环境中,SPE NPs 通过其细菌靶向苯硼酸基团暴露并自发聚集在细胞内细菌周围。酸性细菌微环境会进一步引发 SP NPs 和 EGCG 之间的硼酸酯键断裂,从而使 EGCG 在细菌反应下释放出来,在细胞内发挥局部抗菌作用。MM@SPE NPs 在精确消除细胞内细菌方面的功效在两种大鼠细胞内细菌感染模型中得到了验证。这种级联靶向响应系统为治疗细胞内细菌感染提供了新的解决方案,同时将耐药性风险降至最低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
文献相关原料
公司名称
产品信息
麦克林
3-Aminopropyltriethoxysilane (APTES)
麦克林
Tetraethyl orthosilicate (TEOS)
麦克林
Sodium dodecyl sulfate (SDS)
麦克林
Hexadecyl trimethyl ammonium bromide (CTAB)
阿拉丁
1-(3-Dimethylaminopropyl)?3-ethylcarbodiimide hydrochloride (EDC)
阿拉丁
N-Hydroxysuccinimide (NHS)
阿拉丁
N,N-Dimethylformamide (DMF)
阿拉丁
Triethanolamine (TEA)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


