The involvement of epidermal growth factor receptor/protein kinase B signaling in the tumor intrinsic PD-L1-induced malignant potential of oral squamous cell carcinoma
Abstract
Background
Various antigen-presenting cells and tumor cells-expressing PD-L1 inhibits antitumor immune responses in the tumor microenvironment. Recently, numerous studies have shown that tumor cell intrinsic PD-L1 also plays important roles in tumor growth and progression. On the other hand, oral squamous cell carcinoma (OSCC) cells overexpress epidermal growth factor receptor (EGFR) and EGFR signal pathway exacerbates tumor progression. Therefore, this study assessed whether tumor-intrinsic PD-L1 facilitates malignant potential of OSCC cells through regulation of EGFR signaling.
Methods
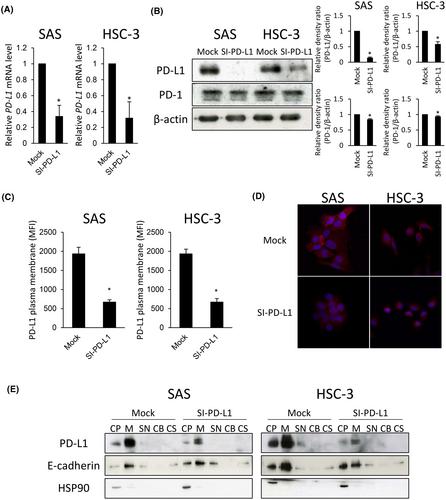
Two OSCC cell lines, SAS and HSC-3, were transfected with PD-L1 and EGFR-specific small interfering RNA (siRNA). Influences of PD-L1 knockdown on malignant potentials of OSCC cells were examined by Cell Counting kit-8 assay, transwell assay, sphere formation assay, flow cytometry, and Western blot. Effects of PD-L1 and EGFR knockdown on each expression were examined by quantitative real-time PCR (qRT-PCR), Western blot, and flow cytometry.
Results
Transfection of an PD-L1-siRNA into OSCC cells decreased the abilities of proliferation, stemness, and mobility of these cells significantly. PD-L1 knockdown also decreased EGFR expression through the promotion of proteasome- and lysosome-mediated degradation and following activation of the EGFR/protekin kinase B (AKT) signal pathway. Meanwhile, EGFR knockdown did not influence PD-L1 expression in SAS and HSC-3 cells, but treatment with a recombinant human EGF induced its expression. Treatment with erlotinib and cetuximab suppressed rhEGF-induced PD-L1 expression and localization in the cellular membrane of both OSCC cells.
Conclusion
OSCC cells-expressing PD-L1 induced by EGF stimulation may promote malignancy intrinsically via the activation of the EGFR/AKT signaling cascade.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


