Direct precipitation of siderite in ferruginous environments
IF 3.7
1区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
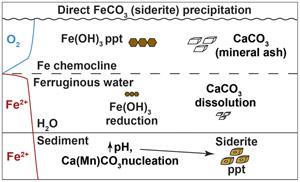
Siderite (FeCO3) is often assumed to precipitate from dissimilatory reduction of Fe-(oxyhydr)oxides, but geochemical and mineralogical analyses from ferruginous (anoxic, Fe-rich) Canyon Lake, USA, suggest Fe-carbonate represents a direct early precipitate unrelated to substantial oxide burial. X-ray absorption near edge structure (XANES) spectroscopy of sediment trap materials and an anoxic sediment core indicated a mixture of Fe(II) and Fe(III) in water column particulates and ferruginous surface sediments, while all Mn phases were reduced. About 60 cm below the sediment–water interface, Fe-Mn carbonates were detected by X-ray diffraction and XANES, while Fe extended X-ray absorption fine structure (EXAFS) spectra were best fit with combinations of a biogenic Fe-oxyhydroxide (“Bio Fe”), greigite, and siderite. Sediment Fe speciation indicates a large pool of reduced Fe with a minor component of oxides. Although we found no evidence of significant carbonate phases above or below the 60 cm horizon, equilibrium modelling indicates siderite supersaturation throughout surface sediment porewater, with pH as the primary control on supersaturation. We conclude that delivery of wildfire ash to sediments increased pH, initiating siderite precipitation from ferruginous porewater.
铁矿环境中菱铁矿的直接沉淀
菱铁矿(FeCO3)通常被认为是由铁(氧氢)氧化物的异纤还原沉淀而成,但对美国峡谷湖(缺氧、富含铁)进行的地球化学和矿物学分析表明,碳酸铁是一种与大量氧化物埋藏无关的早期直接沉淀物。对沉积物捕集材料和缺氧沉积物岩芯进行的 X 射线吸收近缘结构(XANES)光谱分析表明,水柱颗粒和铁锈质表层沉积物中含有铁(II)和铁(III)的混合物,而所有锰相均被还原。在沉积物-水界面下约 60 厘米处,通过 X 射线衍射和 XANES 检测到了铁锰碳酸盐,而铁的扩展 X 射线吸收精细结构(EXAFS)光谱与生物成因的铁氧氢氧化物("生物铁")、绿泥石和菱铁矿的组合最为匹配。沉积物中铁的种类表明,沉积物中含有大量还原铁和少量氧化物。虽然我们在 60 厘米地层上下没有发现明显的碳酸盐相,但平衡建模表明菱铁矿在整个表层沉积物孔隙水中处于过饱和状态,pH 值是过饱和的主要控制因素。我们的结论是,野火灰烬向沉积物的输送增加了 pH 值,启动了铁锈色孔隙水的菱铁矿沉淀。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Geochemical Perspectives Letters
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
7.00
自引率
2.00%
发文量
42
审稿时长
15 weeks
期刊介绍:
Geochemical Perspectives Letters is an open access, internationally peer-reviewed journal of the European Association of Geochemistry (EAG) that publishes short, highest-quality articles spanning geochemical sciences. The journal aims at rapid publication of the most novel research in geochemistry with a focus on outstanding quality, international importance, originality, and stimulating new developments across the vast array of geochemical disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


