Biocatalytic, enantioenriched primary amination of tertiary C–H bonds
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Intermolecular functionalization of tertiary C–H bonds to construct fully substituted stereogenic carbon centres represents a formidable challenge: without the assistance of directing groups, state-of-the-art catalysts struggle to introduce chirality to racemic tertiary sp3-carbon centres. Direct asymmetric functionalization of such centres is a worthy reactivity and selectivity goal for modern biocatalysis. Here we present an engineered nitrene transferase (P411-TEA-5274), derived from a bacterial cytochrome P450, that is capable of aminating tertiary C–H bonds to provide chiral α-tertiary primary amines with high efficiency (up to 2,300 total turnovers) and selectivity (up to >99% enantiomeric excess). The construction of fully substituted stereocentres with methyl and ethyl groups underscores the enzyme’s remarkable selectivity. A comprehensive substrate scope study demonstrates the biocatalyst’s compatibility with diverse functional groups and tertiary C–H bonds. Mechanistic studies explain how active-site residues distinguish between the enantiomers and enable the enzyme to perform this transformation with excellent enantioselectivity. Direct stereoselective amination of tertiary C–H bonds without the assistance of directing groups is a challenging task in synthetic organic chemistry. Now a nitrene transferase is engineered to aminate tertiary C–H bonds with high enantioselectivity, providing direct access to valuable chiral α-tertiary primary amines.
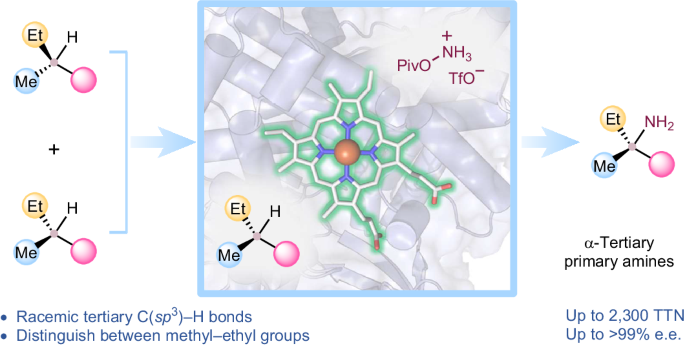
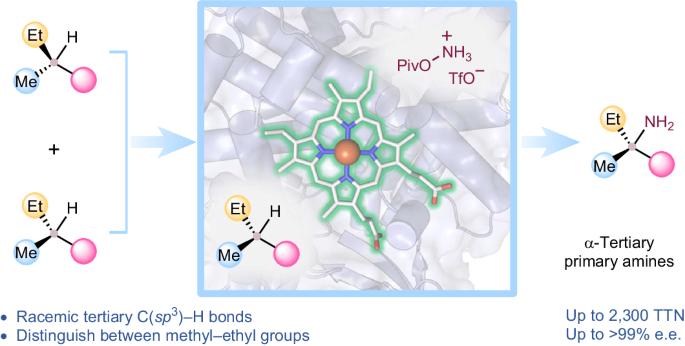
三级 C-H 键的生物催化对映体一级胺化反应
对三级 C-H 键进行分子间官能化以构建完全取代的立体碳中心是一项艰巨的挑战:如果没有定向基团的帮助,最先进的催化剂很难为外消旋三级 sp3 碳中心引入手性。此类中心的直接不对称官能化是现代生物催化中一个值得追求的反应性和选择性目标。在这里,我们展示了一种源自细菌细胞色素 P450 的工程化芘转移酶 (P411-TEA-5274),它能够以高效率(总周转次数高达 2300 次)和高选择性(对映体过量率高达 99%)胺化三级 C-H 键,从而提供手性 α-三级伯胺。构建带有甲基和乙基基团的完全取代立体中心凸显了该酶的显著选择性。一项全面的底物范围研究表明,该生物催化剂与各种官能团和三级 C-H 键兼容。机理研究解释了活性位点残基是如何区分对映体并使酶以出色的对映选择性完成这种转化的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


