Structural basis of TRPV1 modulation by endogenous bioactive lipids
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
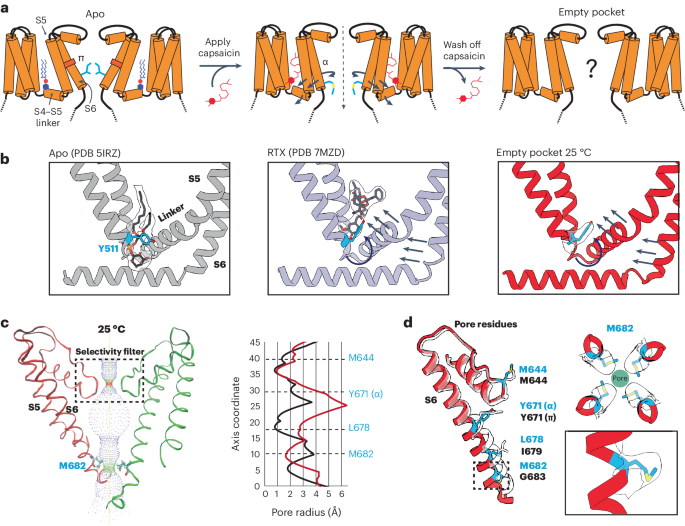
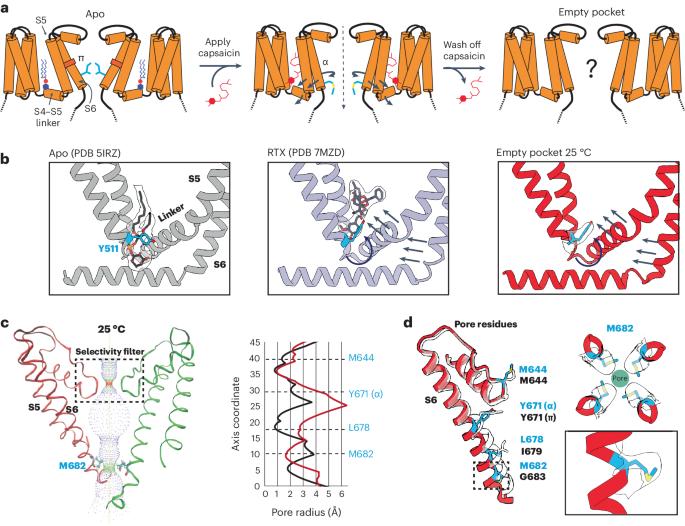
TRP ion channels are modulated by phosphoinositide lipids, but the underlying structural mechanisms remain unclear. The capsaicin- and heat-activated receptor, TRPV1, has served as a model for deciphering lipid modulation, which is relevant to understanding how pro-algesic agents enhance channel activity in the setting of inflammatory pain. Identification of a pocket within the TRPV1 transmembrane core has provided initial clues as to how phosphoinositide lipids bind to and regulate the channel. Here we show that this regulatory pocket in rat TRPV1 can accommodate diverse lipid species, including the inflammatory lipid lysophosphatidic acid, whose actions are determined by their specific modes of binding. Furthermore, we show that an empty-pocket channel lacking an endogenous phosphoinositide lipid assumes an agonist-like state, even at low temperature, substantiating the concept that phosphoinositide lipids serve as negative TRPV1 modulators whose ejection from the binding pocket is a critical step toward activation by thermal or chemical stimuli. Using cryo-EM, the authors elucidate the mechanisms of TRPV1 regulation by bioactive lipids, namely phosphoinositides and the inflammatory lipid lysophosphatidic acid.
内源性生物活性脂质调节 TRPV1 的结构基础
TRP离子通道受磷脂调节,但其基本结构机制仍不清楚。辣椒素和热激活受体 TRPV1 是解密脂质调节的模型,这与了解促镇痛剂如何在炎症性疼痛中增强通道活性有关。对 TRPV1 跨膜核心内一个口袋的鉴定为磷脂如何结合并调节通道提供了初步线索。在这里,我们展示了大鼠 TRPV1 的这一调节袋可容纳多种脂质,包括炎症脂质溶血磷脂酸,其作用由其特定的结合模式决定。此外,我们还发现,即使在低温条件下,缺乏内源性磷脂的空口袋通道也会呈现类似于激动剂的状态,这证实了磷脂作为 TRPV1 负调制剂的概念,从结合口袋中排出磷脂是热刺激或化学刺激激活通道的关键一步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


