A deconstruction–reconstruction strategy for pyrimidine diversification
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Structure–activity relationship (SAR) studies are fundamental to drug and agrochemical development, yet only a few synthetic strategies apply to the nitrogen heteroaromatics frequently encountered in small molecule candidates1–3. Here we present an alternative approach in which we convert pyrimidine-containing compounds into various other nitrogen heteroaromatics. Transforming pyrimidines into their corresponding N-arylpyrimidinium salts enables cleavage into a three-carbon iminoenamine building block, used for various heterocycle-forming reactions. This deconstruction–reconstruction sequence diversifies the initial pyrimidine core and enables access to various heterocycles, such as azoles4. In effect, this approach allows heterocycle formation on complex molecules, resulting in analogues that would be challenging to obtain by other methods. We anticipate that this deconstruction–reconstruction strategy will extend to other heterocycle classes. We present a synthetic method that transforms complex pyrimidine-containing structures into iminoenamines and then uses de novo heterocycle synthesis to obtain substituted pyrimidine and 1,2-azole analogues.
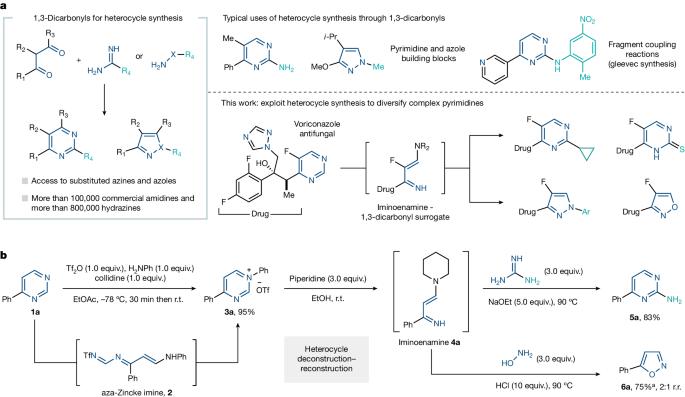
嘧啶多样化的解构-重构战略
结构-活性关系(SAR)研究是药物和农用化学品开发的基础,但只有少数合成策略适用于小分子候选化合物中经常出现的氮杂芳族化合物。将嘧啶转化为相应的 N-芳基嘧啶鎓盐,可以裂解成三碳亚胺烯胺结构单元,用于各种杂环形成反应。这种解构-重构序列使最初的嘧啶核心多样化,并可获得各种杂环,如唑类4。实际上,这种方法可在复杂分子上形成杂环,从而获得其他方法难以获得的类似物。我们预计这种解构-重构策略将扩展到其他杂环类。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


