Tolerogenic dendritic cell-mediated regulatory T cell differentiation by Chinese herbal formulation attenuates colitis progression
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Ulcerative colitis (UC) is a chronic inflammatory disease characterized by loss of immune tolerance to luminal antigens and progressive intestinal tissue injury. Thus, the re-establishment of immune tolerance is crucial for suppressing aberrant immune responses and UC progression. This study aimed to investigate the mechanisms underlying the action of CDD-2103 and its bioactive compounds in mediating immune regulation in mouse models of colitis. Two experimental colitis models, chronic 2,4,6-trinitrobenzene sulfonic acid (TNBS)- and T-cell transfer-induced mice, were used to determine the effects of CDD-2103 on colitis progression. Single-cell transcriptome analysis was used to profile the immune landscape and its interactions after CDD-2103 treatment. Liquid chromatography-mass spectrometry (LC-MS) was used to analyze the major components interacting with lymphoid cells. A primary cell co-culture system was used to confirm the effects of bioactive component. CDD-2103 dose-dependently suppresses the progression of colitis induced by chemicals or T cell transplantation in mice. The effect of CDD-2103 is primarily attributable to an increase in the generation of regulatory T cells (Tregs) in the lamina propria (LP). Single-cell transcriptomic analysis revealed that CDD-2103 treatment increased the number of tolerogenic dendritic cells (DCs). Mechanistically, CDD-2103 promoted tolerogenic DCs accumulation and function by upregulating several genes in the electron transport chain related to oxidative phosphorylation, leading to increased differentiation of Tregs. Further LC-MS analysis identified several compounds in CDD-2103, particularly those distributed within the mesenteric lymph nodes of mice. Subsequent studies revealed that palmatine and berberine promoted tolerogenic bone marrow-derived dendritic cells (BMDC)-mediated Treg differentiation. Overall, our study demonstrated that the clinically beneficial effect of CDD-2103 in the treatment of UC is based on the induction of immune tolerance. In addition, this study supports berberine and palmatine as potential chemical entities in CDD-2103 that modulate immune tolerance.
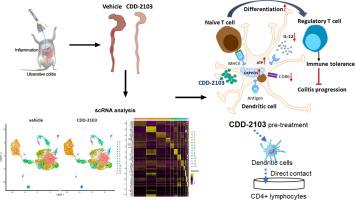
中药配方通过树突状细胞介导的耐受性调节性 T 细胞分化缓解结肠炎进展
溃疡性结肠炎(UC)是一种慢性炎症性疾病,其特点是对管腔抗原的免疫耐受丧失和进行性肠组织损伤。因此,重建免疫耐受对抑制异常免疫反应和 UC 进展至关重要。本研究旨在探讨 CDD-2103 及其生物活性化合物在小鼠结肠炎模型中介导免疫调节的作用机制。研究使用了两种实验性结肠炎模型,即慢性2,4,6-三硝基苯磺酸(TNBS)小鼠和T细胞转移诱导小鼠,以确定CDD-2103对结肠炎进展的影响。单细胞转录组分析被用来描述 CDD-2103 治疗后的免疫格局及其相互作用。液相色谱-质谱法(LC-MS)用于分析与淋巴细胞相互作用的主要成分。使用原代细胞共培养系统确认生物活性成分的作用。CDD-2103剂量依赖性地抑制了化学物质或T细胞移植诱导的小鼠结肠炎的发展。CDD-2103的作用主要归因于固有膜(LP)中调节性T细胞(Tregs)生成的增加。单细胞转录组分析显示,CDD-2103治疗增加了耐受性树突状细胞(DC)的数量。从机理上讲,CDD-2103通过上调与氧化磷酸化相关的电子传递链中的几个基因促进了耐受性树突状细胞的积累和功能,从而导致Tregs的分化增加。进一步的 LC-MS 分析确定了 CDD-2103 中的几种化合物,尤其是分布在小鼠肠系膜淋巴结中的化合物。随后的研究发现,巴马汀和小檗碱促进了由耐受性骨髓树突状细胞(BMDC)介导的Treg分化。总之,我们的研究表明,CDD-2103 治疗 UC 的临床疗效是基于诱导免疫耐受。此外,本研究还支持小檗碱和巴马汀作为 CDD-2103 中调节免疫耐受的潜在化学实体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


