Targeted desialylation and cytolysis of tumour cells by fusing a sialidase to a bispecific T-cell engager
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
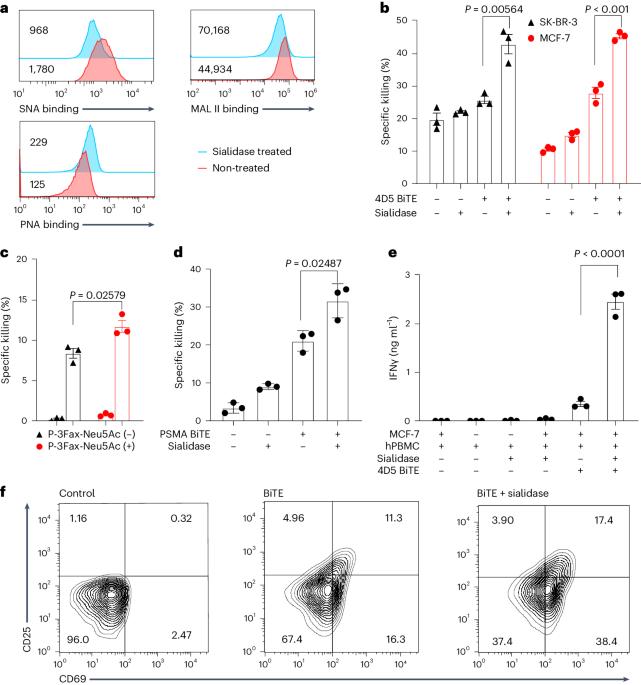
Bispecific T-cell engagers (BiTEs) bring together tumour cells and cytotoxic T cells by binding to specific cell-surface tumour antigens and T-cell receptors, and have been clinically successful for the treatment of B-cell malignancies. Here we show that a BiTE–sialidase fusion protein enhances the susceptibility of solid tumours to BiTE-mediated cytolysis of tumour cells via targeted desialylation—that is, the removal of terminal sialic acid residues on glycans—at the BiTE-induced T-cell–tumour-cell interface. In xenograft and syngeneic mouse models of leukaemia and of melanoma and breast cancer, and compared with the parental BiTE molecules, targeted desialylation via the BiTE–sialidase fusion proteins enhanced the formation of immunological synapses, T-cell activation and T-cell-mediated tumour-cell cytolysis in the presence of the target antigen. The targeted desialylation of tumour cells may enhance the potency of therapies relying on T-cell engagers. The removal of terminal sialic acid residues on glycans at the T-cell–tumour-cell interface via a sialidase fused to a bispecific T-cell engager enhances the susceptibility of solid cancers to T-cell-mediated cytolysis.
通过将硅糖苷酶与双特异性 T 细胞吸引子融合,对肿瘤细胞进行靶向去硅糖苷化和细胞溶解
双特异性T细胞吞噬因子(BiTE)通过与特异性细胞表面肿瘤抗原和T细胞受体结合,将肿瘤细胞和细胞毒性T细胞结合在一起,在临床上成功治疗了B细胞恶性肿瘤。在这里,我们展示了 BiTE-硅糖苷酶融合蛋白通过靶向去硅烷基化--即在 BiTE 诱导的 T 细胞-肿瘤细胞界面上去除聚糖上的末端硅酸残基--来提高实体瘤对 BiTE 介导的肿瘤细胞溶解的敏感性。在白血病、黑色素瘤和乳腺癌的异种移植和合成小鼠模型中,与亲代 BiTE 分子相比,通过 BiTE-sialidase 融合蛋白进行的靶向脱ialylation 能在靶抗原存在的情况下增强免疫突触的形成、T 细胞活化和 T 细胞介导的肿瘤细胞细胞溶解。对肿瘤细胞进行有针对性的脱硅烷基化可增强依赖于T细胞吸引剂的疗法的效力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


