Epithelial–mesenchymal transition in tissue repair and degeneration
IF 81.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Epithelial–mesenchymal transitions (EMTs) are the epitome of cell plasticity in embryonic development and cancer; during EMT, epithelial cells undergo dramatic phenotypic changes and become able to migrate to form different tissues or give rise to metastases, respectively. The importance of EMTs in other contexts, such as tissue repair and fibrosis in the adult, has become increasingly recognized and studied. In this Review, we discuss the function of EMT in the adult after tissue damage and compare features of embryonic and adult EMT. Whereas sustained EMT leads to adult tissue degeneration, fibrosis and organ failure, its transient activation, which confers phenotypic and functional plasticity on somatic cells, promotes tissue repair after damage. Understanding the mechanisms and temporal regulation of different EMTs provides insight into how some tissues heal and has the potential to open new therapeutic avenues to promote repair or regeneration of tissue damage that is currently irreversible. We also discuss therapeutic strategies that modulate EMT that hold clinical promise in ameliorating fibrosis, and how precise EMT activation could be harnessed to enhance tissue repair. During embryonic epithelial–mesenchymal transition, epithelial cells undergo substantial phenotypic changes and acquire migration capacity. This Review compares embryonic and adult non-cancer EMTs and discusses the role of EMTs in adult tissue repair and fibrosis, highlighting therapeutic opportunities to modulate EMT to reduce fibrosis and promote repair.
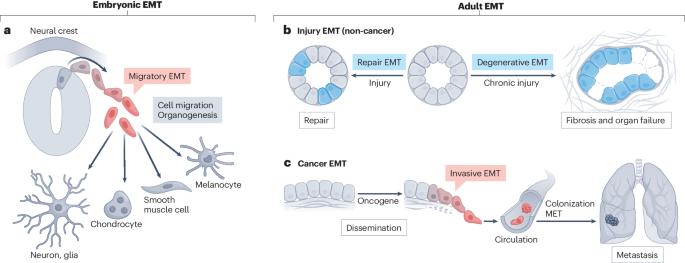
组织修复和退化过程中的上皮-间质转化
上皮-间充质转化(EMT)是胚胎发育和癌症中细胞可塑性的缩影;在 EMT 过程中,上皮细胞会发生剧烈的表型变化,并能迁移到不同的组织中或发生转移。EMT在其他方面的重要性,如成人的组织修复和纤维化,已得到越来越多的认可和研究。在本综述中,我们将讨论成人组织损伤后 EMT 的功能,并比较胚胎和成人 EMT 的特征。持续的EMT会导致成人组织变性、纤维化和器官衰竭,而EMT的短暂激活则会赋予体细胞表型和功能可塑性,促进损伤后的组织修复。了解不同 EMT 的机制和时间调控有助于深入了解某些组织是如何愈合的,并有可能开辟新的治疗途径,促进目前不可逆转的组织损伤的修复或再生。我们还讨论了在改善纤维化方面具有临床前景的调节 EMT 的治疗策略,以及如何利用精确的 EMT 激活来加强组织修复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


