Engineering the cellulolytic bacterium, Clostridium thermocellum, to co-utilize hemicellulose
Abstract
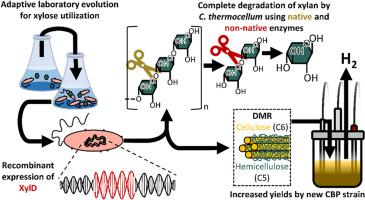
Consolidated bioprocessing (CBP) of lignocellulosic biomass holds promise to realize economic production of second-generation biofuels/chemicals, and Clostridium thermocellum is a leading candidate for CBP due to it being one of the fastest degraders of crystalline cellulose and lignocellulosic biomass. However, CBP by C. thermocellum is approached with co-cultures, because C. thermocellum does not utilize hemicellulose. When compared with a single-species fermentation, the co-culture system introduces unnecessary process complexity that may compromise process robustness. In this study, we engineered C. thermocellum to co-utilize hemicellulose without the need for co-culture. By evolving our previously engineered xylose-utilizing strain in xylose, an evolved clonal isolate (KJC19-9) was obtained and showed improved specific growth rate on xylose by ∼3-fold and displayed comparable growth to a minimally engineered strain grown on the bacteria's naturally preferred substrate, cellobiose. To enable full xylan deconstruction to xylose, we recombinantly expressed three different β-xylosidase enzymes originating from Thermoanaerobacterium saccharolyticum into KJC19-9 and demonstrated growth on xylan with one of the enzymes. This recombinant strain was capable of co-utilizing cellulose and xylan simultaneously, and we integrated the β-xylosidase gene into the KJC19-9 genome, creating the KJCBXint strain. The strain, KJC19-9, consumed monomeric xylose but accumulated xylobiose when grown on pretreated corn stover, whereas the final KJCBXint strain showed significantly greater deconstruction of xylan and xylobiose. This is the first reported C. thermocellum strain capable of degrading and assimilating hemicellulose polysaccharide while retaining its cellulolytic capabilities, unlocking significant potential for CBP in advancing the bioeconomy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


