Remote Follow-up in a Heart Failure Pragmatic Trial: Insights From the CONNECT-HF
IF 6.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Randomized controlled trials typically require study-specific visits, which can burden participants and sites. Remote follow-up, such as centralized call centers for participant-reported or site-reported, holds promise for reducing costs and enhancing the pragmatism of trials. In this secondary analysis of the CONNECT-HF (Care Optimization Through Patient and Hospital Engagement For HF) trial, we aimed to evaluate the completeness and validity of the remote follow-up process.
Methods and Results
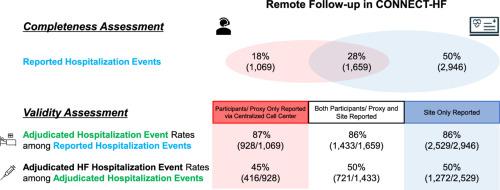
The CONNECT-HF trial evaluated the effect of a post-discharge quality-improvement intervention for heart failure compared to usual care for up to 1 year. Suspected events were reported either by participants or by health care proxies through a centralized call center or by sites through medical-record queries. When potential hospitalization events were suspected, additional medical records were collected and adjudicated. Among 5942 potential hospitalizations, 18% were only participant-reported, 28% were reported by both participants and sites, and 50% were only site-reported. Concordance rates between the participant/site reports and adjudication for hospitalization were high: 87% participant-reported, 86% both, and 86% site-reported. Rates of adjudicated heart failure hospitalization events among adjudicated all-cause hospitalization were lower but also consistent: 45% participant-reported, 50% both, and 50% site-reported.
Conclusions
Participant-only and site-only reports missed a substantial number of hospitalization events. We observed similar concordance between participant/site reports and adjudication for hospitalizations. Combining participant-reported and site-reported outcomes data is important to capture and validate hospitalizations effectively in pragmatic heart failure trials.
心力衰竭实用性试验中的远程随访:来自 CONNECT-HF 的启示。
背景随机对照试验通常需要对特定研究进行访问,这会给参与者和研究机构造成负担。远程随访,如通过集中呼叫中心进行参与者报告或研究机构报告,有望降低成本并提高试验的实用性。在这项 CONNECT-HF(通过患者和医院参与优化心力衰竭护理)试验的二次分析中,我们旨在评估远程随访过程的完整性和有效性。方法和结果CONNECT-HF 试验评估了出院后心力衰竭质量改善干预与常规护理相比长达 1 年的效果。疑似事件由参与者或医疗代理通过中央呼叫中心报告,或由医疗机构通过医疗记录查询报告。当怀疑可能发生住院事件时,会收集更多的医疗记录并进行判定。在 5942 例潜在住院事件中,18% 仅由参与者报告,28% 由参与者和医疗点共同报告,50% 仅由医疗点报告。参与者/医疗点报告与住院裁定之间的一致率很高:参与者报告占 87%,参与者和医疗点报告均占 86%,医疗点报告占 86%。在裁定的全因住院事件中,裁定的心衰住院事件比率较低,但也是一致的:结论仅参与者报告和仅现场报告遗漏了大量住院事件。我们观察到参与者/现场报告与住院裁定之间具有相似的一致性。结合参与者报告和研究机构报告的结果数据对于在实用性心衰试验中有效捕捉和验证住院事件非常重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Cardiac Failure
医学-心血管系统
CiteScore
7.80
自引率
8.30%
发文量
653
审稿时长
21 days
期刊介绍:
Journal of Cardiac Failure publishes original, peer-reviewed communications of scientific excellence and review articles on clinical research, basic human studies, animal studies, and bench research with potential clinical applications to heart failure - pathogenesis, etiology, epidemiology, pathophysiological mechanisms, assessment, prevention, and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


