Cannabidiol suppresses proliferation and induces cell death, autophagy and senescence in human cholangiocarcinoma cells via the PI3K/AKT/mTOR pathway
IF 3
3区 医学
Q1 INTEGRATIVE & COMPLEMENTARY MEDICINE
Journal of Traditional and Complementary Medicine
Pub Date : 2024-04-17
DOI:10.1016/j.jtcme.2024.04.007
引用次数: 0
Abstract
Background and aim
Cholangiocarcinoma (CCA) is usually diagnosed at a late stage, leading to treatment failure. Cannabidiol (CBD), exhibits diverse anti-cancer effects in various cancers, offering avenues for improving CCA treatment. This study investigated the effects of CBD on human CCA cells and the underlying mechanisms in vitro and in vivo.
Experimental procedure
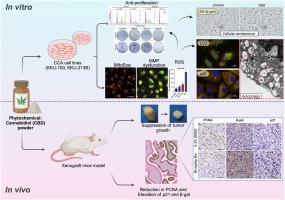
The effects of CBD on three CCA cell lines (KKU-213B, KKU-100, KKU-055) were assessed using the SRB assay, clonogenic assay, cell cycle arrest, and 3D holotomography. Morphological changes were examined using transmission electron microscopy, while mitochondrial ROS levels and mitochondrial membrane potential were studied using MitoSOX, JC-1, and DCFH-DA. Cellular senescence induction was evaluated via SA-β-gal staining. Protein associatedwith autophagy and cellular senescence were analyzed using Western blot and/or immunofluorescent assays. A xenograft model demonstrated the anti-tumor activity of CBD and the induction of cellular senescence through immunohistochemistry targeting PCNA, β-gal, and p21.
Results and conclusion
CBD effectively inhibited CCA cell proliferation, suppressed colony formation and induced G0/G1 phase cell cycle arrest. Morphological examination revealed lipid droplets/vesicles in CCA cell lines. CBD induced autophagy by upregulating LC3BII, downregulating p62, and inhibiting the p-PI3K, p-AKT, and p-mTOR pathways. Additionally, CBD disrupted mitochondrial homeostasis by elevating ROS, reducing membrane potential, and induced cellular senescence by increasing the expression of p53 and p21. In-vitro results were confirmed by xenograft models. Overall, CBD suppresses proliferation and induces cell death, autophagy and senescence in CCA cells via the PI3K/AKT/mTOR pathway, which indicates a therapeutic option for CCA treatment.
大麻二酚通过 PI3K/AKT/mTOR 通路抑制人胆管癌细胞的增殖并诱导细胞死亡、自噬和衰老
背景和目的胆管癌(CCA)通常在晚期才被诊断出来,导致治疗失败。大麻二酚(CBD)在多种癌症中表现出不同的抗癌作用,为改善 CCA 的治疗提供了途径。本研究调查了 CBD 对人类 CCA 细胞的影响及其在体外和体内的潜在机制。实验过程 CBD 对三种 CCA 细胞系(KKU-213B、KKU-100 和 KKU-055)的影响通过 SRB 试验、克隆生成试验、细胞周期停滞和三维全图进行评估。透射电子显微镜检查了形态学变化,MitoSOX、JC-1 和 DCFH-DA 研究了线粒体 ROS 水平和线粒体膜电位。细胞衰老诱导通过 SA-β-gal 染色进行评估。通过 Western 印迹和/或免疫荧光检测分析了与自噬和细胞衰老相关的蛋白质。通过针对 PCNA、β-gal 和 p21 的免疫组化,异种移植模型证明了 CBD 的抗肿瘤活性和细胞衰老诱导作用。形态学检查显示,CCA 细胞系中存在脂滴/囊泡。CBD 通过上调 LC3BII、下调 p62 以及抑制 p-PI3K、p-AKT 和 p-mTOR 通路来诱导自噬。此外,CBD 还通过提高 ROS、降低膜电位来破坏线粒体的平衡,并通过增加 p53 和 p21 的表达来诱导细胞衰老。体外实验结果在异种移植模型中得到了证实。总之,CBD 可通过 PI3K/AKT/mTOR 通路抑制 CCA 细胞增殖并诱导细胞死亡、自噬和衰老,为 CCA 治疗提供了一种治疗选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Traditional and Complementary Medicine
Medicine-Complementary and Alternative Medicine
CiteScore
9.30
自引率
6.70%
发文量
78
审稿时长
66 days
期刊介绍:
eJTCM is committed to publish research providing the biological and clinical grounds for using Traditional and Complementary Medical treatments as well as studies that demonstrate the pathophysiological and molecular/biochemical bases supporting the effectiveness of such treatments. Review articles are by invitation only.
eJTCM is receiving an increasing amount of submission, and we need to adopt more stringent criteria to select the articles that can be considered for peer review. Note that eJTCM is striving to increase the quality and medical relevance of the publications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


