Real-world use of long-acting cabotegravir and rilpivirine: 12-month results of the inJectable Antiretroviral therapy feasiBility Study (JABS)
Abstract
Objectives
The inJectable Antiretroviral feasiBility Study (JABS) aimed to evaluate the implementation of long-acting regimens in a ‘real world’ Australian setting, with inclusion of participants with complex medical needs, social vulnerability and/or historical non-adherence.
Methods
JABS was a 12-month, single-centre, single-arm, open-label phase IV study of long-acting cabotegravir 600 mg plus rilpivirine 900 mg administered intramuscularly every 2 months to adults with treated HIV-1 infection. The primary endpoint was the proportion of attendances and administration of injections within a 14-day dosing window over 12 months, out of the total prescribed doses. Secondary endpoints included proportions of missed appointments, use of oral bridging, discontinuations, virological failures, adverse events and participant-reported outcomes. A multidisciplinary adherence programme embedded in the clinical service known as REACH provided support to JABS participants.
Results
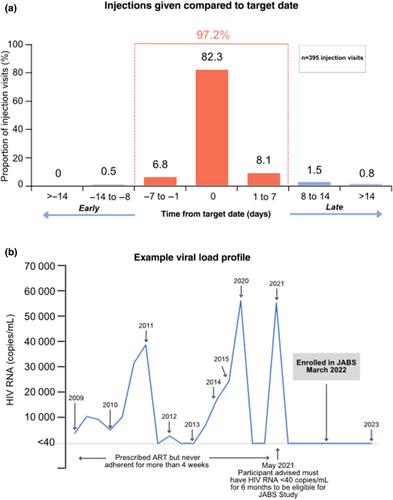
Of 60 participants enrolled by May 2022, 60% had one or more complexity or vulnerability factors identified, including absence of social supports (50%), mental health issues, alcohol or drug use (30%) and financial hardship or instability (13%), among others. Twenty-seven per cent of participants had historical non-adherence to antiretroviral therapy. Out of 395 prescribed doses, 97.2% of injections were administered within correct dosing windows at clinic visits. Two courses of short-term oral bridging were required. The rate of injection site reactions was 29%, the majority being grade 1–2. There were no virological failures, no serious adverse events and only one injection-related study discontinuation. High baseline treatment satisfaction and acceptability of injections increased by month 12. Those with vulnerability factors had similar adherence to injections as those without such factors. Ninety-eight per cent of the participants who completed 12 months on the study have maintained long-acting therapy, virological suppression and retention in care.
Conclusions
Long-acting cabotegravir plus rilpivirine was associated with very high adherence, maintenance of virological suppression, safety and treatment satisfaction in a diverse Australian clinic population, comparable to results of phase III randomized clinical trials. Individuals with vulnerability factors can achieve adherence to injections with individualized support. Long-acting therapies in this group can increase the subsequent engagement in clinical care.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


