Autologous HER2-specific CAR T cells after lymphodepletion for advanced sarcoma: a phase 1 trial
IF 23.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
In this prospective, interventional phase 1 study for individuals with advanced sarcoma, we infused autologous HER2-specific chimeric antigen receptor T cells (HER2 CAR T cells) after lymphodepletion with fludarabine (Flu) ± cyclophosphamide (Cy): 1 × 108 T cells per m2 after Flu (cohort A) or Flu/Cy (cohort B) and 1 × 108 CAR+ T cells per m2 after Flu/Cy (cohort C). The primary outcome was assessment of safety of one dose of HER2 CAR T cells after lymphodepletion. Determination of antitumor responses was the secondary outcome. Thirteen individuals were treated in 14 enrollments, and seven received multiple infusions. HER2 CAR T cells expanded after 19 of 21 infusions. Nine of 12 individuals in cohorts A and B developed grade 1–2 cytokine release syndrome. Two individuals in cohort C experienced dose-limiting toxicity with grade 3–4 cytokine release syndrome. Antitumor activity was observed with clinical benefit in 50% of individuals treated. The tumor samples analyzed showed spatial heterogeneity of immune cells and clustering by sarcoma type and by treatment response. Our results affirm HER2 as a CAR T cell target and demonstrate the safety of this therapeutic approach in sarcoma. ClinicalTrials.gov registration: NCT00902044 . In this phase 1 trial, Hegde et al. treat 13 individuals with advanced sarcoma with lymphodepletion followed by HER2-specific CAR T cells, which were found to be safe and showed antitumor activity.
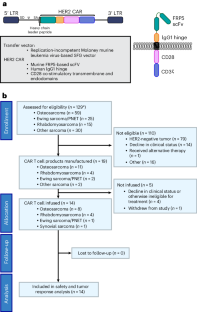
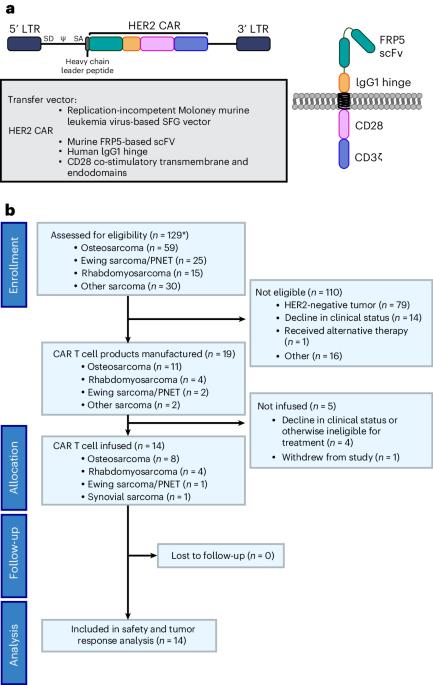
晚期肉瘤淋巴切除术后的自体 HER2 特异性 CAR T 细胞:1 期试验。
在这项针对晚期肉瘤患者的前瞻性、干预性 1 期研究中,我们在使用氟达拉滨(Flu)±环磷酰胺(Cy)进行淋巴清除后输注了自体 HER2 特异性嵌合抗原受体 T 细胞(HER2 CAR T 细胞):在使用氟达拉滨(Flu)±环磷酰胺(Cy)进行淋巴清除后,每平方米使用 1 × 108 个 T 细胞(A 组)或 Flu/Cy(B 组);在使用 Flu/Cy 后,每平方米使用 1 × 108 个 CAR+ T 细胞(C 组)。主要结果是评估淋巴清除后一剂 HER2 CAR T 细胞的安全性。次要结果是确定抗肿瘤反应。14人中有13人接受了治疗,其中7人接受了多次输注。HER2 CAR T细胞在21次输注中的19次后扩增。A 组和 B 组的 12 人中有 9 人出现了 1-2 级细胞因子释放综合征。C组中有两人出现了剂量限制性毒性,即3-4级细胞因子释放综合征。50%的受试者观察到了抗肿瘤活性,并获得了临床疗效。分析的肿瘤样本显示了免疫细胞的空间异质性,并按肉瘤类型和治疗反应进行了分组。我们的研究结果肯定了 HER2 是 CAR T 细胞的靶点,并证明了这种治疗方法在肉瘤中的安全性。ClinicalTrials.gov 注册:NCT00902044 。在这项 1 期试验中,Hegde 等人对 13 名晚期肉瘤患者进行了淋巴清扫,然后使用 HER2 特异性 CAR T 细胞进行治疗,结果发现这种治疗方法是安全的,而且具有抗肿瘤活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


