Solar-light-activated periodate for degradation and detoxification of highly toxic 6PPD-quinone at environmental levels
引用次数: 0
Abstract
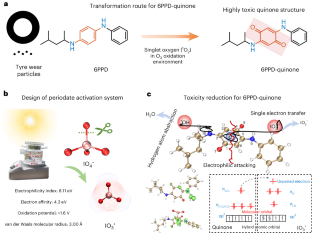
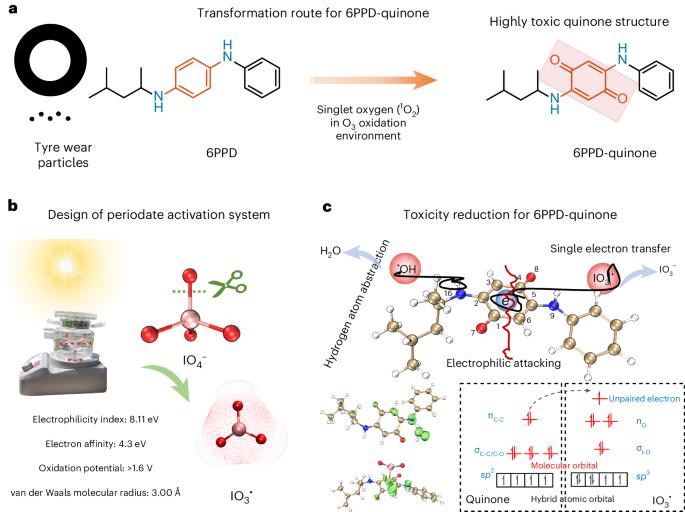
Degradation and detoxication of highly toxic 6PPD-quinone remain great challenges due to its stable structure. Here we establish a solar-light-driven IO4− activation system for efficient degradation of 6PPD-quinone at environmental concentration levels (10–100 μg l−1), with residual concentration below 5.7 ng l−1 (detection limit) within 30 min. IO3• was determined as the primary reactive species after IO4− activation for cleavage of the highly toxic quinone structure. Single electron transfer is the most favourable route for IO3• attacking, in which single electrons achieve self-driven transfer from 6PPD-quinone to IO3• due to the maintenance of spatial inversion symmetry generated by dipole moments. Femtosecond transient absorption spectra confirmed the formation of 6PPD-quinone cationic radical (6PPD-quinone•+), which was the key reaction intermediate. This study proposes a promising technology for degradation and detoxification of highly toxic 6PPD-quinone in water and brings deep insight into the reaction mechanism within IO4− activation systems. There is a pressing need to develop effective treatment technologies for 6PPD-quinone, a newly discovered micropollutant, given its prevalent presence in water. The proposed advanced oxidation of IO4− activation under solar light irradiation achieves efficient degradation of 6PPD-quinone at environmental concentration levels.
太阳光激活的高碘酸盐在环境水平上降解和解毒剧毒 6PPD-quinone
由于高毒性 6PPD-quinone 结构稳定,其降解和解毒仍然是一项巨大挑战。在这里,我们建立了一个太阳光驱动的 IO4- 活化系统,可在环境浓度水平(10-100 μg l-1)下高效降解 6PPD-quinone,30 分钟内残留浓度低于 5.7 ng l-1(检测限)。IO3- 被确定为 IO4- 激活后裂解剧毒醌结构的主要反应物。单电子转移是 IO3- 攻击的最有利途径,其中由于偶极矩产生的空间反转对称性的维持,单电子实现了从 6PPD-quinone 到 IO3- 的自驱动转移。飞秒瞬态吸收光谱证实了关键反应中间体 6PPD-quinone 阳离子自由基(6PPD-quinone-+)的形成。这项研究为降解和解毒水中的剧毒 6PPD-quinone 提出了一种前景广阔的技术,并深入揭示了 IO4- 活化体系中的反应机理。6PPD-quinone 是一种新发现的微污染物,鉴于其在水中的普遍存在,迫切需要开发有效的处理技术。在太阳光照射下,拟议的 IO4-活化高级氧化技术可在环境浓度水平上实现 6PPD-quinone 的高效降解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


