Candidate reference measurement procedure for serum 17-hydroxyprogesterone quantification via isotope dilution liquid chromatography–tandem mass spectrometry
Abstract
Background
Accurate quantification of 17-hydroxyprogesterone (17-OHP) in serum is vital for clinical and research applications. However, inter-laboratory variability in test results exists owing to the lack of a standardized reference measurement procedure (RMP). Therefore, in this study, we developed a highly accurate, cost-effective, and user-friendly candidate RMP (cRMP) for analyzing 17-OHP in serum.
Methods
We quantified 17-OHP in serum using a one-step liquid–liquid extraction method with the addition of 17-OHP-13C3, followed by liquid chromatography–tandem mass spectrometry. The ability of these methods to suppress interference was evaluated by chromatographic analysis. We assessed accuracy, specificity, the lower limit of quantitation, linearity, and matrix effects by following the standards specified by the Clinical and Laboratory Standards Institute. The consistency between the developed cRMP and the chemiluminescence method was evaluated through experiments with 120 clinical samples.
Results
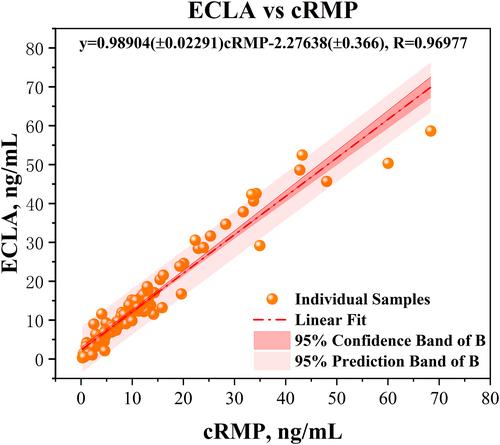
The developed cRMP required only 8 min for accurate quantification of serum 17-OHP without bias from matrix effects or interference from 19 metabolites added as potential interferents. The method exhibited favorable measurement performance, with a quantitation limit of 0.086 ng/mL, linear range of 0.1–400 ng/mL, a total imprecision of ≤2.90%, spike recovery of 100.1%–100.6%, and relative deviations from assigned target values (RfB Institution) of −2.91% to 1.10%. The cRMP demonstrated good consistency with the conventional assay (chemiluminescence method), with a correlation coefficient R of 0.96977.
Conclusion
A cRMP with high accuracy, cost-effectiveness, and convenient operation was developed for quantifying 17-OHP in serum. Its simplicity and robust performance make it an invaluable addition to the arsenal of analytical tools available for laboratories. This method can enhance the accuracy and reliability of 17-OHP measurements across various laboratories.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


