The use of methanol as a C1 building block
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Methanol is a key building block in the chemical industry. In recent years, it has been used as a C1 source in various organic transformations in the presence of a transition-metal catalyst. This protocol describes the ruthenium- and cobalt-catalyzed utilization of methanol in different types of methylation reactions and heterocycle synthesis. Initially, we describe the synthesis of tridentate ligands (L1–L3) and their corresponding Ru(II) complexes (Ru-1, -2 and -3) and then detail how to apply these Ru(II) complexes and Co/PP3 (PP3 = P(CH2CH2PPh2)3) in various methanol dehydrogenative coupling reactions. We discuss six types of transformations by using methanol or a methanol/water mixture. The experimental setup for all the catalytic reactions is similar and involves adding all the respective reagents and solvents to an argon-filled pressure tube, which is sealed (by screw cap) and refluxed at the indicated temperature before the desired products are isolated and characterized. The catalytic systems described in this protocol work well for both small-scale and preparative-scale synthesis of various N-methylated amines/amides, C-methylated products and quinazolinones. These catalytic reactions are greener and more sustainable than conventional synthesis methods, with only H2 and/or H2O as by-products, and we evaluate the ‘green chemistry metrics’ for a typical substrate. The total time required for the catalytic experiments described in this protocol is 16–28 h, and the operation time is 4 h. An average level of expertise in organic synthesis is required to carry out these protocols. This protocol details methods for using methanol in methylation reactions, including the synthesis of suitable transition metal-containing catalysts, and in the synthesis of heterocycles. The methods described produce only H2 and H2O as by-products.
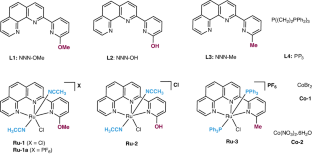
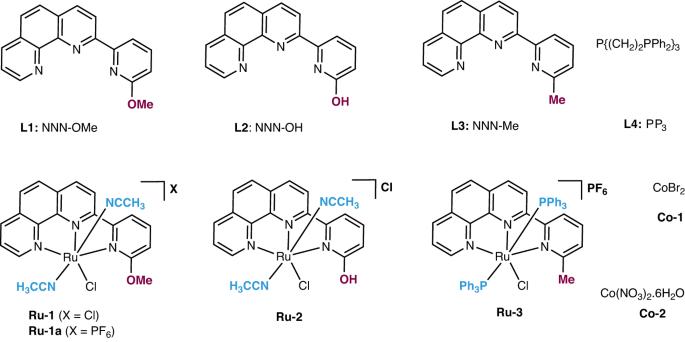
使用甲醇作为 C1 的组成部分。
甲醇是化学工业的重要组成部分。近年来,在过渡金属催化剂的作用下,甲醇被用作各种有机转化的 C1 源。本规程介绍了钌和钴催化甲醇在不同类型甲基化反应和杂环合成中的应用。首先,我们介绍了三叉配体(L1-L3)及其相应的 Ru(II)配合物(Ru-1、-2 和 -3)的合成,然后详细介绍了如何在各种甲醇脱氢偶联反应中应用这些 Ru(II)配合物和 Co/PP3(PP3 = P(CH2CH2PPh2)3)。我们讨论了使用甲醇或甲醇/水混合物进行的六种类型的转化。所有催化反应的实验装置都很相似,都是将所有相应的试剂和溶剂加入充满氩气的压力管中,然后密封(用螺旋盖)并在指定温度下回流,最后分离出所需产物并对其进行表征。本方案中描述的催化系统在小规模和制备规模合成各种 N-甲基化胺/酰胺、C-甲基化产物和喹唑啉酮时都非常有效。与传统合成方法相比,这些催化反应更环保、更可持续,副产物仅为 H2 和/或 H2O。本方案中描述的催化实验所需总时间为 16-28 小时,操作时间为 4 小时。本规程详细介绍了将甲醇用于甲基化反应(包括合成合适的含过渡金属催化剂)和合成杂环 的方法。所述方法只产生 H2 和 H2O 作为副产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


