Role of oxygen vacancies in CO2 methanation over zirconia: A mechanistic DFT and microkinetic study
Abstract
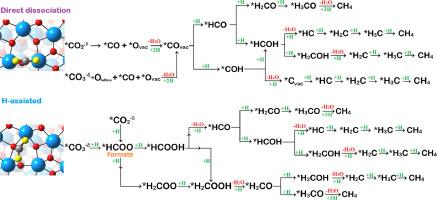
The use of CO2 as a source of carbon for energy-storage through their transformation into methane represents an attractive approach in modern days. The development of stable and active catalysts as well as a deeper understanding of reaction mechanisms is essential in that field. Herein, the CO2 methanation reaction over zirconia was thoroughly investigated via DFT calculations and microkinetic modeling. The catalytic surface was represented by a monoclinic ZrO2 surface where CO2 adsorption and elementary hydrogenation steps have been systematically explored. It was demonstrated that the participation of an active oxygen vacancy (reduced surface) is crucial to activate the stable bonds of CO2 for further hydrogenation steps; the stoichiometric surface has a minor contribution to CH4 production. Two main reaction pathways were investigated: i) CO2 dissociation with later hydrogenations; (ii) H-assisted (early hydrogenation) mechanisms. It was demonstrated that the latter is kinetically favorable passing through a formate intermediate. Microkinetic simulations indicate that the initial adsorption configuration plays a central role to methane formation: more weakly adsorbed geometry happens to be more important. Thus, the present findings provide new insights and critical information on the role of oxygen vacancies to methanation mechanisms.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


