Receptor-recognition and antiviral mechanisms of retrovirus-derived human proteins
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
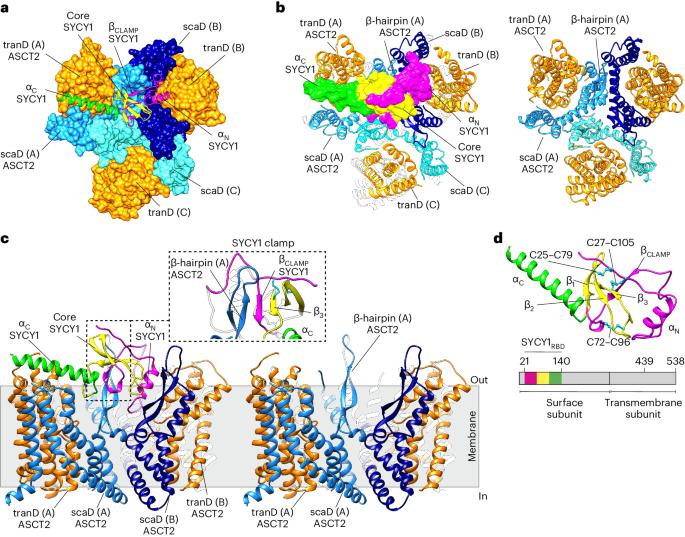
Human syncytin-1 and suppressyn are cellular proteins of retroviral origin involved in cell–cell fusion events to establish the maternal–fetal interface in the placenta. In cell culture, they restrict infections from members of the largest interference group of vertebrate retroviruses, and are regarded as host immunity factors expressed during development. At the core of the syncytin-1 and suppressyn functions are poorly understood mechanisms to recognize a common cellular receptor, the membrane transporter ASCT2. Here, we present cryo-electron microscopy structures of human ASCT2 in complexes with the receptor-binding domains of syncytin-1 and suppressyn. Despite their evolutionary divergence, the two placental proteins occupy similar positions in ASCT2, and are stabilized by the formation of a hybrid β-sheet or ‘clamp’ with the receptor. Structural predictions of the receptor-binding domains of extant retroviruses indicate overlapping binding interfaces and clamping sites with ASCT2, revealing a competition mechanism between the placental proteins and the retroviruses. Our work uncovers a common ASCT2 recognition mechanism by a large group of endogenous and disease-causing retroviruses, and provides high-resolution views on how placental human proteins exert morphological and immunological functions. The structures of the retrovirus-derived human syncytin-1 and suppressyn in complexes with their shared receptor reveal an ancient cellular recognition mechanism that underlies key morphological and immunological functions in placenta.
逆转录病毒衍生人类蛋白质的受体识别和抗病毒机制
人类 syncytin-1 和 suppressyn 是源自逆转录病毒的细胞蛋白,参与细胞-细胞融合事件,在胎盘中建立母胎界面。在细胞培养中,它们限制了脊椎动物逆转录病毒最大干扰群成员的感染,被认为是发育过程中表达的宿主免疫因子。syncytin-1 和 suppressyn 功能的核心是识别共同细胞受体(膜转运体 ASCT2)的机制,但人们对这一机制知之甚少。在这里,我们展示了人类 ASCT2 与 syncytin-1 和 suppressyn 的受体结合结构域复合物的冷冻电镜结构。尽管这两种胎盘蛋白在进化过程中存在差异,但它们在 ASCT2 中占据相似的位置,并通过与受体形成混合 β 片层或 "钳夹 "而稳定下来。对现存逆转录病毒受体结合结构域的结构预测表明,与ASCT2的结合界面和钳位重叠,揭示了胎盘蛋白与逆转录病毒之间的竞争机制。我们的研究揭示了一大类内源性逆转录病毒和致病逆转录病毒对ASCT2的共同识别机制,并提供了关于人类胎盘蛋白如何发挥形态学和免疫学功能的高分辨率视图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


