Formylation boosts the performance of light-driven overcrowded alkene-derived rotary molecular motors
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Artificial molecular motors and machines constitute a critical element in the transition from individual molecular motion to the creation of collective dynamic molecular systems and responsive materials. The design of artificial light-driven molecular motors operating with high efficiency and selectivity constitutes an ongoing fundamental challenge. Here we present a highly versatile synthetic approach based on Rieche formylation that boosts the quantum yield of the forward photoisomerization reaction while reaching near-perfect selectivity in the steps involved in the unidirectional rotary cycle and drastically reducing competing photoreactions. This motor is readily accessible in its enantiopure form and operates with nearly quantitative photoconversions. It can easily be functionalized further and outperforms its direct predecessor as a reconfigurable chiral dopant in cholesteric liquid crystal materials. Overcrowded alkene-derived molecular motors convert light and heat into chirality-directed unidirectional rotary motion, but the efficiency of their photochemical isomerization remains limited. Now formylation of the motor core has been shown to boost all aspects of motor photochemistry by improving photochemical efficiency, diminishing competing processes and redshifting absorption.
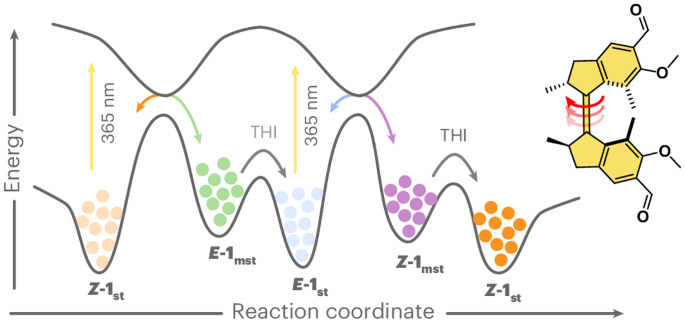
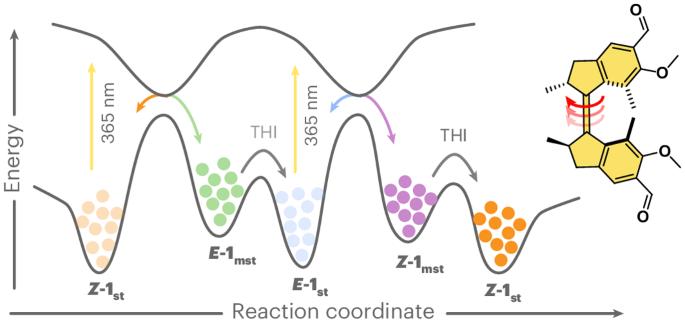
甲酰化提高了光驱动的过度拥挤烯基旋转分子马达的性能
人造分子马达和机器是从单个分子运动过渡到创造集体动态分子系统和反应材料的关键要素。设计高效率、高选择性的人工光驱动分子马达是一项持续的基础挑战。在这里,我们提出了一种基于 Rieche 甲酰化的多功能合成方法,它可以提高正向光异构化反应的量子产率,同时在单向旋转循环所涉及的步骤中达到近乎完美的选择性,并大大减少竞争性光反应。这种马达的对映体纯度很高,几乎可以实现定量光转化。作为胆甾液晶材料中的一种可重新配置的手性掺杂剂,它可以很容易地进一步官能化,而且性能优于其直接前身。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


