H-bond promoted NPh3-mediated SrTiO3-photocatalyzed cascade decarboxylative coupling/annulation of benzo[d]isothiazole 1,1-dioxides
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The first applying SrTiO3 semiconductor as a photocatalyst in organic synthesis and using NPh3 as a redox catalyst in photocatalysis was reported. With SrTiO3 as the heterogeneous photocatalyst, NPh3 as the homogeneous redox catalyst, H-bond as the promoter, paraformaldehyde as the cheap and atom economic methylene source, various 1,2,3,9b-tetrahydrobenzo[d]imidazo[1,5-b]isothiazole 5,5-dioxides can be efficiently synthesized through the visible-light-induced cascade decarboxylative coupling/annulation reaction.
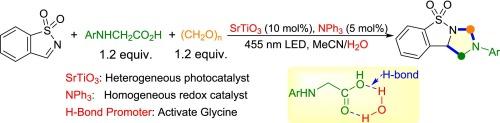
H 键促进 NPh3 介导的 SrTiO3 光催化级联苯并[d]异噻唑 1,1-二氧杂环的脱羧偶联/annulation
该研究首次将 SrTiO3 半导体作为光催化剂应用于有机合成,并将 NPh3 作为光催化中的氧化还原催化剂。以 SrTiO3 为异相光催化剂,NPh3 为均相氧化还原催化剂,H-键为促进剂,对甲醛为廉价且原子经济的亚甲基源,通过可见光诱导的级联脱羧偶联/环化反应,可高效合成各种 1,2,3,9b- 四氢苯并[d]咪唑并[1,5-b]异噻唑 5,5- 二氧杂化物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


