Transient loss of Polycomb components induces an epigenetic cancer fate
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
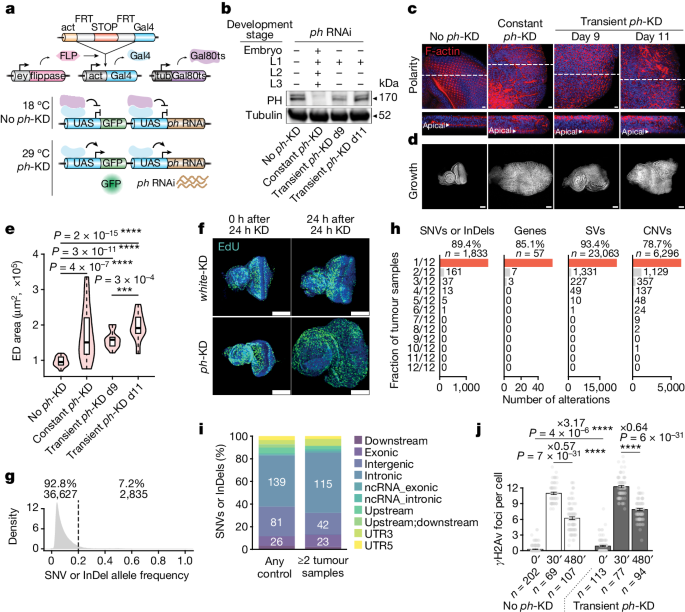
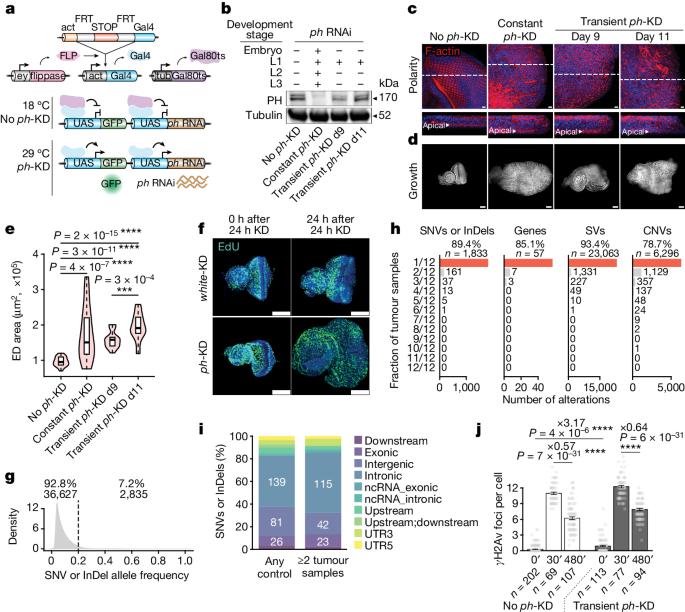
Although cancer initiation and progression are generally associated with the accumulation of somatic mutations1,2, substantial epigenomic alterations underlie many aspects of tumorigenesis and cancer susceptibility3–6, suggesting that genetic mechanisms might not be the only drivers of malignant transformation7. However, whether purely non-genetic mechanisms are sufficient to initiate tumorigenesis irrespective of mutations has been unknown. Here, we show that a transient perturbation of transcriptional silencing mediated by Polycomb group proteins is sufficient to induce an irreversible switch to a cancer cell fate in Drosophila. This is linked to the irreversible derepression of genes that can drive tumorigenesis, including members of the JAK–STAT signalling pathway and zfh1, the fly homologue of the ZEB1 oncogene, whose aberrant activation is required for Polycomb perturbation-induced tumorigenesis. These data show that a reversible depletion of Polycomb proteins can induce cancer in the absence of driver mutations, suggesting that tumours can emerge through epigenetic dysregulation leading to inheritance of altered cell fates. A transient perturbation of transcriptional silencing mediated by Polycomb proteins is sufficient to induce an epigenetic cancer cell fate in Drosophila in the absence of driver mutations.
瞬时丧失多聚核糖体成分会诱发表观遗传学癌症命运
虽然癌症的发生和发展通常与体细胞突变的积累有关1,2,但肿瘤发生和癌症易感性的许多方面都与大量表观基因组改变有关3,4,5,6,这表明遗传机制可能不是恶性转化的唯一驱动因素7。然而,无论基因突变与否,纯粹的非遗传机制是否足以启动肿瘤发生一直是个未知数。在这里,我们发现,由多聚核糖体组蛋白介导的转录沉默的短暂扰动足以诱导果蝇不可逆地转入癌细胞命运。这与可驱动肿瘤发生的基因的不可逆抑制有关,这些基因包括 JAK-STAT 信号通路的成员和 ZEB1 致癌基因的蝇类同源物 zfh1。这些数据表明,在没有驱动基因突变的情况下,Polycomb 蛋白的可逆损耗可诱发癌症,这表明肿瘤可通过表观遗传失调导致细胞命运改变而出现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


